DHEA Protects Human Cholangiocytes and Hepatocytes against Apoptosis and Oxidative Stress
- PMID: 35326489
- PMCID: PMC8947473
- DOI: 10.3390/cells11061038
DHEA Protects Human Cholangiocytes and Hepatocytes against Apoptosis and Oxidative Stress
Abstract
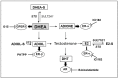
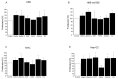
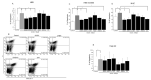
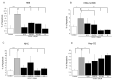
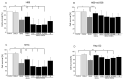
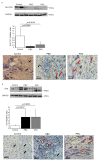
Primary biliary cholangitis (PBC) is a rare chronic cholestatic and immune-mediated liver disease of unknown aetiology that targets intrahepatic bile duct cells (cholangiocytes) and primarily affects postmenopausal women, when their estrogen levels sharply decrease. An impaired cholangiocyte response to estrogen characterizes the terminal stage of the disease, as this is when an inefficiency of cholangiocyte proliferation, in balancing the loss of intrahepatic bile ducts, is observed. Here, we report that the estrogen precursor dehydroepiandrosterone (DHEA) and its sulfate metabolites, DHEA-S and 17 β-estradiol, enhance the proliferation of cholangiocytes and hepatocytes in vitro. Flow cytometry analysis showed that DHEA and DHEA-S decreased glyco-chenodeoxycholic acid (GCDC)-driven apoptosis in cholangiocytes. Cell viability assay (MTT) indicated that ER-α, -β, and the G-protein-coupled estrogen receptor, are involved in the protection of DHEA against oxidative stress in cholangiocytes. Finally, immunoblot analysis showed an elevated level of steroid sulfatase and a reduced level of sulfotransferase 1E1 enzymes, involved in the desulfation/sulfation process of estrogens in cirrhotic PBC, and primary sclerosis cholangitis (PSC) liver tissues, another type of chronic cholestatic and immune-mediated liver disease. Taken together, these results suggest that DHEA can prevent the deleterious effects of certain potentially toxic bile acids and reactive oxygen species, delaying the onset of liver disease.
Keywords: DHEA; apoptosis; cholangiocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Alvaro D., Invernizzi P., Onori P., Franchitto A., De Santis A., Crosignani A., Sferra R., Ginanni-Corradini S., Mancino M.G., Maggioni M., et al. Estrogen receptors in cholangiocytes and the progression of primary biliary cirrhosis. J. Hepatol. 2004;41:905–912. doi: 10.1016/j.jhep.2004.08.022. - DOI - PubMed
-
- Teng Y., Radde B.N., Litchfield L.M., Ivanova M.M., Prough R.A., Clark B.J., Doll M.A., Hein D.W., Klinge C.M. Dehydroepiandrosterone Activation of G-protein-coupled Estrogen Receptor Rapidly Stimulates MicroRNA-21 Transcription in Human Hepatocellular Carcinoma Cells. J. Biol. Chem. 2015;290:15799–15811. doi: 10.1074/jbc.M115.641167. - DOI - PMC - PubMed
-
- Van Erpecum K.J., Janssens A.R., Kreuning J., Ruiter D.J., Kroon H.M., Grond A.J. Generalized peliosis hepatis and cirrhosis after long-term use of oral contraceptives. Am. J. Gastroenterol. 1988;83:572–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

