Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes
- PMID: 35326497
- PMCID: PMC8947267
- DOI: 10.3390/cells11061045
Cardiac Ischemia On-a-Chip: Antiarrhythmic Effect of Levosimendan on Ischemic Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Abstract
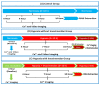
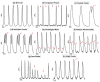
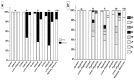
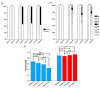
Ischemic heart disease (IHD) is one of the leading causes of mortality worldwide. Preserving functionality and preventing arrhythmias of the heart are key principles in the management of patients with IHD. Levosimendan, a unique calcium (Ca2+) enhancer with inotropic activity, has been introduced into clinical usage for heart failure treatment. Human-induced pluripotent cell-derived cardiomyocytes (hiPSC-CMs) offer an opportunity to better understand the pathophysiological mechanisms of the disease as well as to serve as a platform for drug screening. Here, we developed an in vitro IHD model using hiPSC-CMs in hypoxic conditions and defined the effects of the subsequent hypoxic stress on CMs functionality. Furthermore, the effect of levosimendan on hiPSC-CMs functionality was evaluated during and after hypoxic stress. The morphology, contractile, Ca2+-handling, and gene expression properties of hiPSC-CMs were investigated in response to hypoxia. Hypoxia resulted in significant cardiac arrhythmia and decreased Ca2+ transient amplitude. In addition, disorganization of sarcomere structure was observed after hypoxia induction. Interestingly, levosimendan presented significant antiarrhythmic properties, as the arrhythmia was abolished or markedly reduced with levosimendan treatment either during or after the hypoxic stress. Moreover, levosimendan presented significant protection from the sarcomere alterations induced by hypoxia. In conclusion, this chip model appears to be a suitable preclinical representation of IHD. With this hypoxia platform, detailed knowledge of the disease pathophysiology can be obtained. The antiarrhythmic effect of levosimendan was clearly observed, suggesting a possible new clinical use for the drug.
Keywords: antiarrhythmic effect; calcium transient; cardiac ischemia on-a-chip; human-induced pluripotent stem cell-derived cardiomyocytes; ischemic heart disease; levosimendan.
Conflict of interest statement
The authors declare no conflict of interest. A patent application related to this work has been submitted.
Figures





References
-
- Oh J.G., Ishikawa K. Experimental Models of Cardiovascular Diseases. Springer; New York, NY, USA: 2018. Experimental models of cardiovascular diseases: Overview; pp. 3–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

