The Role of CD200-CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation
- PMID: 35326506
- PMCID: PMC8947338
- DOI: 10.3390/cells11061055
The Role of CD200-CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation
Abstract
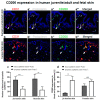
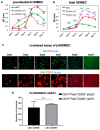
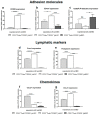
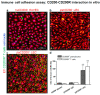
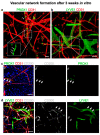
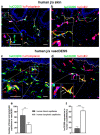
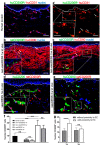
CD200 is a cell membrane glycoprotein that interacts with its structurally related receptor (CD200R) expressed on immune cells. We characterized CD200-CD200R interactions in human adult/juvenile (j/a) and fetal (f) skin and in in vivo prevascularized skin substitutes (vascDESS) prepared by co-culturing human dermal microvascular endothelial cells (HDMEC), containing both blood (BEC) and lymphatic (LEC) EC. We detected the highest expression of CD200 on lymphatic capillaries in j/a and f skin as well as in vascDESS in vivo, whereas it was only weakly expressed on blood capillaries. Notably, the highest CD200 levels were detected on LEC with enhanced Podoplanin expression, while reduced expression was observed on Podoplanin-low LEC. Further, qRT-PCR analysis revealed upregulated expression of some chemokines, including CC-chemokine ligand 21 (CCL21) in j/aCD200+ LEC, as compared to j/aCD200- LEC. The expression of CD200R was mainly detected on myeloid cells such as granulocytes, monocytes/macrophages, T cells in human peripheral blood, and human and rat skin. Functional immunoassays demonstrated specific binding of skin-derived CD200+ HDMEC to myeloid CD200R+ cells in vitro. Importantly, we confirmed enhanced CD200-CD200R interaction in vascDESS in vivo. We concluded that the CD200-CD200R axis plays a crucial role in regulating tissue inflammation during skin wound healing.
Keywords: CD200 (OX2); CD200 receptor; angiogenesis–immune cells/myeloid cells; blood capillaries; lymphatic capillaries; microvascular endothelial cells; regenerative medicine; skin bioengineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Expression Profile of CD157 Reveals Functional Heterogeneity of Capillaries in Human Dermal Skin.Biomedicines. 2022 Mar 15;10(3):676. doi: 10.3390/biomedicines10030676. Biomedicines. 2022. PMID: 35327478 Free PMC article.
-
CD200-CD200R Pathway in the Regulation of Tumor Immune Microenvironment and Immunotherapy.Adv Exp Med Biol. 2020;1223:155-165. doi: 10.1007/978-3-030-35582-1_8. Adv Exp Med Biol. 2020. PMID: 32030689 Free PMC article. Review.
-
CD200:CD200R Interactions and Their Importance in Immunoregulation.Int J Mol Sci. 2021 Feb 5;22(4):1602. doi: 10.3390/ijms22041602. Int J Mol Sci. 2021. PMID: 33562512 Free PMC article. Review.
-
Characterization of the CD200 receptor family in mice and humans and their interactions with CD200.J Immunol. 2003 Sep 15;171(6):3034-46. doi: 10.4049/jimmunol.171.6.3034. J Immunol. 2003. PMID: 12960329
-
Regulation of myeloid cell function through the CD200 receptor.J Immunol. 2006 Jan 1;176(1):191-9. doi: 10.4049/jimmunol.176.1.191. J Immunol. 2006. PMID: 16365410
Cited by
-
Per1/Per2 knockout Affects Spleen Immune Function in Elderly Mice via Inducing Spleen Lymphocyte Ferroptosis.Int J Mol Sci. 2022 Oct 26;23(21):12962. doi: 10.3390/ijms232112962. Int J Mol Sci. 2022. PMID: 36361751 Free PMC article.
-
Breathing new life into tissue engineering: exploring cutting-edge vascularization strategies for skin substitutes.Angiogenesis. 2024 Nov;27(4):587-621. doi: 10.1007/s10456-024-09928-6. Epub 2024 Jun 6. Angiogenesis. 2024. PMID: 38842751 Free PMC article. Review.
-
Single-cell RNA-seq and bulk-seq identify RAB17 as a potential regulator of angiogenesis by human dermal microvascular endothelial cells in diabetic foot ulcers.Burns Trauma. 2023 Aug 18;11:tkad020. doi: 10.1093/burnst/tkad020. eCollection 2023. Burns Trauma. 2023. PMID: 37605780 Free PMC article.
-
Endothelial Cell-Derived Soluble CD200 Determines the Ability of Immune Cells to Cross the Blood-Brain Barrier.Int J Mol Sci. 2024 Aug 27;25(17):9262. doi: 10.3390/ijms25179262. Int J Mol Sci. 2024. PMID: 39273210 Free PMC article.
-
Isolation and Culture of Human Dermal Fibroblasts.Methods Mol Biol. 2025;2922:75-83. doi: 10.1007/978-1-0716-4510-9_6. Methods Mol Biol. 2025. PMID: 40208528
References
-
- Biedermann T., Bottcher-Haberzeth S., Klar A.S., Widmer D.S., Pontiggia L., Weber A.D., Weber D.M., Schiestl C., Meuli M., Reichmann E. The Influence of Stromal Cells on the Pigmentation of Tissue-Engineered Dermo-Epidermal Skin Grafts. Tissue Eng. Part A. 2015;21:960–969. doi: 10.1089/ten.tea.2014.0327. - DOI - PMC - PubMed
-
- Bottcher-Haberzeth S., Klar A.S., Biedermann T., Schiestl C., Meuli-Simmen C., Reichmann E., Meuli M. “Trooping the color”: Restoring the original donor skin color by addition of melanocytes to bioengineered skin analogs. Pediatr. Surg. Int. 2013;29:239–247. doi: 10.1007/s00383-012-3217-0. - DOI - PubMed
-
- Klar A.S., Biedermann T., Michalak K., Michalczyk T., Meuli-Simmen C., Scherberich A., Meuli M., Reichmann E. Human Adipose Mesenchymal Cells Inhibit Melanocyte Differentiation and the Pigmentation of Human Skin via Increased Expression of TGF-beta1. J. Invest. Dermatol. 2017;137:2560–2569. doi: 10.1016/j.jid.2017.06.027. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

