Cumulative Metabolic and Epigenetic Effects of Paternal and/or Maternal Supplementation with Arachidonic Acid across Three Consecutive Generations in Mice
- PMID: 35326508
- PMCID: PMC8947399
- DOI: 10.3390/cells11061057
Cumulative Metabolic and Epigenetic Effects of Paternal and/or Maternal Supplementation with Arachidonic Acid across Three Consecutive Generations in Mice
Abstract
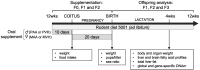
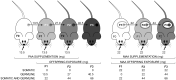
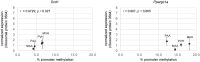
Apart from the known associations between arachidonic acid (AA), weight gain, and neurological and immune function, AA exposure leads to alterations in global and gene-specific DNA methylation (DNAm) and fatty acid (FA) content in human cultured cells. However, it is unknown as to whether the latter effects occur in vivo and are maintained over extended periods of time and across generations. To address this issue, we asked whether AA supplementation for three consecutive generations (prior to coitus in sires or in utero in dams) affected offspring growth phenotypes, in addition to liver DNAm and FA profiles in mice. Twelve-week-old BALB/c mice were exposed daily to AA dissolved in soybean oil (vehicle, VH), or VH only, for 10 days prior to mating or during the entire pregnancy (20 days). On average, 15 mice were supplemented per generation, followed by analysis of offspring body weight and liver traits (x average = 36 and 10 per generation, respectively). Body weight cumulatively increased in F2 and F3 offspring generations and positively correlated with milligrams of paternal or maternal offspring AA exposure. A concomitant increase in liver weight was observed. Notably, akin to AA-challenged cultured cells, global DNAm and cis-7-hexadecenoic acid (16:1n-9), an anti-inflammatory FA that is dependent on stearoyl-CoA desaturase 1 (SCD1) activity, increased with milligrams of AA exposure. In accordance, liver Scd1 promoter methylation decreased with milligrams of germline AA exposure and was negatively correlated with liver weight. Our results show that mice retain cellular memories of AA exposure across generations that could potentially be beneficial to the innate immune system.
Keywords: DNA methylation; arachidonic acid; cis-7-hexadecenoic acid; cumulative; growth; stearoyl-CoA desaturase 1; transgenerational.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Effects of paternal arachidonic acid supplementation on offspring behavior and hypothalamus inflammation markers in the mouse.PLoS One. 2024 Mar 21;19(3):e0300141. doi: 10.1371/journal.pone.0300141. eCollection 2024. PLoS One. 2024. PMID: 38512839 Free PMC article.
-
Intergenerational impact of paternal lifetime exposures to both folic acid deficiency and supplementation on reproductive outcomes and imprinted gene methylation.Mol Hum Reprod. 2017 Jul 1;23(7):461-477. doi: 10.1093/molehr/gax029. Mol Hum Reprod. 2017. PMID: 28535307 Free PMC article.
-
The effects of paternal high-fat diet exposure on offspring metabolism with epigenetic changes in the mouse adiponectin and leptin gene promoters.Am J Physiol Endocrinol Metab. 2016 Jul 1;311(1):E236-45. doi: 10.1152/ajpendo.00095.2016. Epub 2016 May 31. Am J Physiol Endocrinol Metab. 2016. PMID: 27245335
-
NTP technical report on the toxicity studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F1 Mice.Toxic Rep Ser. 1995 Apr;30:1-G5. Toxic Rep Ser. 1995. PMID: 12209194
-
Transgenerational inheritance: how impacts to the epigenetic and genetic information of parents affect offspring health.Hum Reprod Update. 2019 Sep 11;25(5):518-540. doi: 10.1093/humupd/dmz017. Hum Reprod Update. 2019. PMID: 31374565
Cited by
-
A Survey of Fatty Acid Content of the Male Reproductive System in Mice Supplemented With Arachidonic Acid.J Lipids. 2024 Dec 19;2024:3351340. doi: 10.1155/jl/3351340. eCollection 2024. J Lipids. 2024. PMID: 39734583 Free PMC article.
-
NR2F2 Reactivation in Early-life Adipocyte Stem-like Cells Rescues Adipocyte Mitochondrial Oxidation.bioRxiv [Preprint]. 2024 Sep 9:2024.09.09.611047. doi: 10.1101/2024.09.09.611047. bioRxiv. 2024. PMID: 39314382 Free PMC article. Preprint.
-
Transcriptional Factors and Epigenetic Mechanisms in Obesity and Related Metabolic Comorbidities.Cells. 2022 Aug 14;11(16):2520. doi: 10.3390/cells11162520. Cells. 2022. PMID: 36010597 Free PMC article.
-
Fatty acids and epigenetics in health and diseases.Food Sci Biotechnol. 2024 Jul 25;33(14):3153-3166. doi: 10.1007/s10068-024-01664-3. eCollection 2024 Nov. Food Sci Biotechnol. 2024. PMID: 39328231 Free PMC article. Review.
-
Roles of Palmitoleic Acid and Its Positional Isomers, Hypogeic and Sapienic Acids, in Inflammation, Metabolic Diseases and Cancer.Cells. 2022 Jul 8;11(14):2146. doi: 10.3390/cells11142146. Cells. 2022. PMID: 35883589 Free PMC article. Review.
References
-
- Fleming T.P., Watkins A.J., Velazquez M.A., Mathers J.C., Prentice A.M., Stephenson J., Barker M., Saffery R., Yajnik C.S., Eckert J.J., et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet. 2018;391:1842–1852. doi: 10.1016/S0140-6736(18)30312-X. - DOI - PMC - PubMed
-
- Dunford A.R., Sangster J.M. Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: A systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2017;11:S655–S662. doi: 10.1016/j.dsx.2017.04.021. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

