Occurrence of Total and Proteinase K-Resistant Alpha-Synuclein in Glioblastoma Cells Depends on mTOR Activity
- PMID: 35326535
- PMCID: PMC8946689
- DOI: 10.3390/cancers14061382
Occurrence of Total and Proteinase K-Resistant Alpha-Synuclein in Glioblastoma Cells Depends on mTOR Activity
Abstract
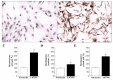
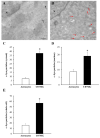
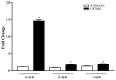
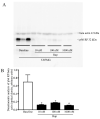
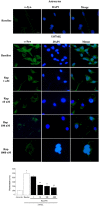
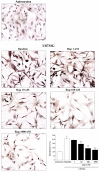
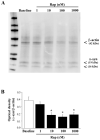
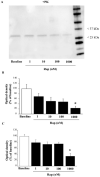
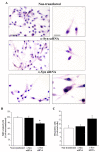
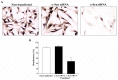
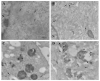
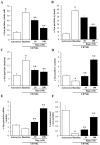
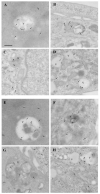
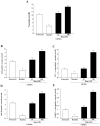
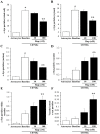
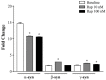
Alpha-synuclein (α-syn) is a protein considered to be detrimental in a number of degenerative disorders (synucleinopathies) of which α-syn aggregates are considered a pathological hallmark. The clearance of α-syn strongly depends on autophagy, which can be stimulated by inhibiting the mechanistic target of rapamycin (mTOR). Thus, the overexpression of mTOR and severe autophagy suppression may produce α-syn accumulation, including the proteinase K-resistant protein isoform. Glioblastoma multiforme (GBM) is a lethal brain tumor that features mTOR overexpression and severe autophagy inhibition. Cell pathology in GBM is reminiscent of a fast, progressive degenerative disorder. Therefore, the present work questions whether, as is analogous to neurons during degenerative disorders, an overexpression of α-syn occurs within GBM cells. A high amount of α-syn was documented in GBM cells via real-time PCR (RT-PCR), Western blotting, immunohistochemistry, immuno-fluorescence, and ultrastructural stoichiometry, compared with the amount of β- and γ-synucleins and compared with the amount of α-syn counted within astrocytes. The present study indicates that (i) α-syn is overexpressed in GBM cells, (ii) α-syn expression includes a proteinase-K resistant isoform, (iii) α-syn is dispersed from autophagy-like vacuoles to the cytosol, (iv) α-syn overexpression and cytosol dispersion are mitigated by rapamycin, and (v) the α-syn-related GBM-like phenotype is mitigated by silencing the SNCA gene.
Keywords: autophagy vacuoles; cell-clearing systems; glioblastoma multiforme; mechanistic target of rapamycin; qRT-PCR; siRNA; synucleins; transmission electron microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Spreading of Alpha Synuclein from Glioblastoma Cells towards Astrocytes Correlates with Stem-like Properties.Cancers (Basel). 2022 Mar 10;14(6):1417. doi: 10.3390/cancers14061417. Cancers (Basel). 2022. PMID: 35326570 Free PMC article.
-
Distinct alpha-Synuclein species induced by seeding are selectively cleared by the Lysosome or the Proteasome in neuronally differentiated SH-SY5Y cells.J Neurochem. 2021 Mar;156(6):880-896. doi: 10.1111/jnc.15174. Epub 2020 Sep 22. J Neurochem. 2021. PMID: 32869336
-
Preserved proteinase K-resistant core after amplification of alpha-synuclein aggregates: Implication to disease-related structural study.Biochem Biophys Res Commun. 2020 Feb 12;522(3):655-661. doi: 10.1016/j.bbrc.2019.11.142. Epub 2019 Nov 28. Biochem Biophys Res Commun. 2020. PMID: 31785806
-
Neuropathology of synuclein aggregates.J Neurosci Res. 2000 Jul 15;61(2):121-7. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. J Neurosci Res. 2000. PMID: 10878583 Review.
-
The good and bad of therapeutic strategies that directly target α-synuclein.IUBMB Life. 2020 Apr;72(4):590-600. doi: 10.1002/iub.2194. Epub 2019 Nov 6. IUBMB Life. 2020. PMID: 31693290 Review.
Cited by
-
Natural Products Targeting Hsp90 for a Concurrent Strategy in Glioblastoma and Neurodegeneration.Metabolites. 2022 Nov 21;12(11):1153. doi: 10.3390/metabo12111153. Metabolites. 2022. PMID: 36422293 Free PMC article. Review.
-
Spreading of Alpha Synuclein from Glioblastoma Cells towards Astrocytes Correlates with Stem-like Properties.Cancers (Basel). 2022 Mar 10;14(6):1417. doi: 10.3390/cancers14061417. Cancers (Basel). 2022. PMID: 35326570 Free PMC article.
-
Autophagy Activation Associates with Suppression of Prion Protein and Improved Mitochondrial Status in Glioblastoma Cells.Cells. 2023 Jan 4;12(2):221. doi: 10.3390/cells12020221. Cells. 2023. PMID: 36672156 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

