Downregulation and Hypermethylation of GABPB1 Is Associated with Aggressive Thyroid Cancer Features
- PMID: 35326537
- PMCID: PMC8946831
- DOI: 10.3390/cancers14061385
Downregulation and Hypermethylation of GABPB1 Is Associated with Aggressive Thyroid Cancer Features
Abstract
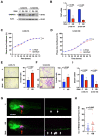
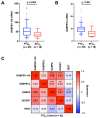
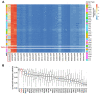
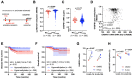
Promoter mutations of the telomerase reverse transcriptase (TERT) gene occur frequently in thyroid carcinoma (TC), including papillary (PTC) and anaplastic subtypes (ATC). Given that the ETS family transcription factors GABPA and GABPB1 activate the mutant TERT promoter and induce TERT expression for telomerase activation, GABPB1 has been proposed as a cancer therapeutic target to inhibit telomerase. Here, we sought to determine the role of GABPB1 in TC pathogenesis. In TC-derived cells carrying the mutated TERT promoter, GABPB1 knockdown led to diminished TERT expression but significantly increased invasive potentials in vitro and metastatic potential in a xenograft zebrafish model and altered expression of markers for epithelial-to-mesenchymal transition. GABPB1 expression was downregulated in aggressive TCs. Low GABPB1 expression correlated with its promoter hypermethylation, which in turn was also associated with shorter disease-free survival. Consistently, DNA methylation inhibitors enhanced GABPB1 expression, as observed upon reduced promoter methylation. Our results suggest that GABPB1 is required for TERT expression and telomerase activation, but itself serves as a tumor suppressor to inhibit TC progression. Furthermore, aberrant DNA methylation leads to GABPB1 silencing, thereby promoting TC aggressiveness. Thus, caution is needed if targeting GABPB1 for cancer therapy is considered.
Keywords: DNA methylation; GABPA; GABPB1; TERT; telomerase; thyroid carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

