Pro-Inflammatory Cytokines Trigger the Overexpression of Tumour-Related Splice Variant RAC1B in Polarized Colorectal Cells
- PMID: 35326545
- PMCID: PMC8946262
- DOI: 10.3390/cancers14061393
Pro-Inflammatory Cytokines Trigger the Overexpression of Tumour-Related Splice Variant RAC1B in Polarized Colorectal Cells
Erratum in
-
Correction: Pereira et al. Pro-Inflammatory Cytokines Trigger the Overexpression of Tumour-Related Splice Variant RAC1B in Polarized Colorectal Cells. Cancers 2022, 14, 1393.Cancers (Basel). 2025 May 13;17(10):1642. doi: 10.3390/cancers17101642. Cancers (Basel). 2025. PMID: 40427229 Free PMC article.
Abstract
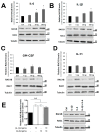
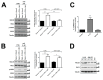
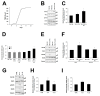
An inflammatory microenvironment is a tumour-promoting condition that provides survival signals to which cancer cells respond with gene expression changes. One example is the alternative splicing variant Rat Sarcoma Viral Oncogene Homolog (Ras)-Related C3 Botulinum Toxin Substrate 1 (RAC1)B, which we previously identified in a subset of V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF)-mutated colorectal tumours. RAC1B was also increased in samples from inflammatory bowel disease patients or in an acute colitis mouse model. Here, we used an epithelial-like layer of polarized Caco-2 or T84 colorectal cancer (CRC) cells in co-culture with fibroblasts, monocytes or macrophages and analysed the effect on RAC1B expression in the CRC cells by RT-PCR, Western blot and confocal fluorescence microscopy. We found that the presence of cancer-associated fibroblasts and M1 macrophages induced the most significant increase in RAC1B levels in the polarized CRC cells, accompanied by a progressive loss of epithelial organization. Under these conditions, we identified interleukin (IL)-6 as the main trigger for the increase in RAC1B levels, associated with Signal Transducer and Activator of Transcription (STAT)3 activation. IL-6 neutralization by a specific antibody abrogated both RAC1B overexpression and STAT3 phosphorylation in polarized CRC cells. Our data identify that pro-inflammatory extracellular signals from stromal cells can trigger the overexpression of tumour-related RAC1B in polarized CRC cells. The results will help to understand the tumour-promoting effect of inflammation and identify novel therapeutic strategies.
Keywords: RAC1; colorectal cancer; inflammation; interleukin; macrophage; signal transduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Velho S., Moutinho C., Cirnes L., Albuquerque C., Hamelin R., Schmitt F., Carneiro F., Oliveira C., Seruca R. BRAF, KRAS and PIK3CA mutations in colorectal serrated polyps and cancer: Primary or secondary genetic events in colorectal carcinogenesis? BMC Cancer. 2008;8:255. doi: 10.1186/1471-2407-8-255. - DOI - PMC - PubMed
-
- Jordan P. Colorectal Cancer Subtypes—The Current Portrait. In: Jordan P., editor. Targeted Therapy of Colorectal Cancer Subtypes. Volome 1110. Springer International Publishing; Cham, Switzerland: 2018. pp. 1–6. Advances in Experimental Medicine and Biology. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

