Emerging Novel Combined CAR-T Cell Therapies
- PMID: 35326556
- PMCID: PMC8945996
- DOI: 10.3390/cancers14061403
Emerging Novel Combined CAR-T Cell Therapies
Abstract
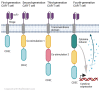
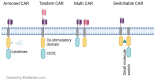
Chimeric antigen receptors (CAR) T cells are T cells engineered to express membrane receptors with high specificity to recognize specific target antigens presented by cancer cells and are co-stimulated with intracellular signals to increase the T cell response. CAR-T cell therapy is emerging as a novel therapeutic approach to improve T cell specificity that will lead to advances in precision medicine. CAR-T cells have had impressive outcomes in hematological malignancies. However, there continue to be significant limitations of these therapeutic responses in targeting solid malignancies such as heterogeneous antigens in solid tumors, tumor immunosuppressive microenvironment, risk of on-target/off-tumor, infiltrating CAR-T cells, immunosuppressive checkpoint molecules, and cytokines. This review paper summarizes recent approaches and innovations through combination therapies of CAR-T cells and other immunotherapy or small molecule drugs to counter the above disadvantages to potentiate the activity of CAR-T cells.
Keywords: T cell; checkpoint inhibitor; chimeric antigen receptor (CAR); combined therapy; hematologic malignancies; immunotherapy; solid tumor; target antigen; tumor-associated antigen (TAA); tumor-specific antigen (TSA).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Jensen M.C., Popplewell L., Cooper L.J., DiGiusto D., Kalos M., Ostberg J.R., Forman S.J. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 2010;16:1245–1256. doi: 10.1016/j.bbmt.2010.03.014. - DOI - PMC - PubMed
-
- Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials