PARP Inhibitors Resistance: Mechanisms and Perspectives
- PMID: 35326571
- PMCID: PMC8945953
- DOI: 10.3390/cancers14061420
PARP Inhibitors Resistance: Mechanisms and Perspectives
Abstract
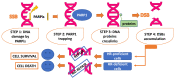
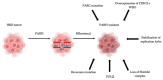
PolyADP-ribose polymerase (PARP) inhibitors (PARPis) represent the first clinically approved drugs able to provoke "synthetic lethality" in patients with homologous recombination-deficient (HRD) tumors. Four PARPis have just received approval for the treatment of several types of cancer. Besides, another three additional PARPis underlying the same mechanism of action are currently under investigation. Despite the success of these targeted agents, the increasing use of PARPis in clinical practice for the treatment of different tumors raised the issue of PARPis resistance, and the consequent disease relapse and dismal prognosis for patients. Several mechanisms of resistance have been investigated, and ongoing studies are currently focusing on strategies to address this challenge and overcome PARPis resistance. This review aims to analyze the mechanisms underlying PARPis resistance known today and discuss potential therapeutic strategies to overcome these processes of resistance in the future.
Keywords: BRCA; DNA damage repair; PARP inhibitor resistance; homologous recombination; ovarian cancer; polyADP-ribose polymerase (PARP) inhibitor; replication fork.
Conflict of interest statement
V.S. reports grants from Clovis Oncology, grants from TESARO, grants from GSK, grants from Astra Zeneca, grants from MSD, grants from PHARMAMAR, grants from EISAI, grants from ROCHE, outside the submitted work; G.S. reports personal fees from ROCHE, personal fees from Clovis Oncology, personal fees from Astra Zeneca, personal fees from PharmaMar, personal fees from Tesaro, outside the submitted work; D.L. reports grants from ROCHE, grants and personal fees from GSK, grants and personal fees from Clovis Oncology, grants and personal fees from MSD, grants from Incyte, grants and personal fees from PHARMAMAR, grants from IMMUNOGEN, grants and personal fees from GENMAB, personal fees from AMGEN, grants and personal fees from Astra Zeneca, outside the submitted work; E.G., M.G., C.R., L.M., M.V.C., V.G., F.C (Floriana Camarda). F.T., C.N. and F.C. (Francesca Ciccarone) have nothing to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

