Emerging Canonical and Non-Canonical Roles of Granzyme B in Health and Disease
- PMID: 35326588
- PMCID: PMC8946077
- DOI: 10.3390/cancers14061436
Emerging Canonical and Non-Canonical Roles of Granzyme B in Health and Disease
Abstract
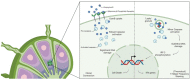
The Granzyme (Gzm) family has classically been recognized as a cytotoxic tool utilized by cytotoxic T lymphocytes (CTL) and natural killer (NK) cells to illicit cell death to infected and cancerous cells. Their importance is established based on evidence showing that deficiencies in these cell death executors result in defective immune responses. Recent findings have shown the importance of Granzyme B (GzmB) in regulatory immune cells, which may contribute to tumor growth and immune evasion during cancer development. Other studies have shown that members of the Gzm family are important for biological processes such as extracellular matrix remodeling, angiogenesis and organized vascular degradation. With this growing body of evidence, it is becoming more important to understand the broader function of Gzm's rather than a specific executor of cell death, and we should be aware of the many alternative roles that Gzm's play in physiological and pathological conditions. Therefore, we review the classical as well as novel non-canonical functions of GzmB and discuss approaches to utilize these new findings to address current gaps in our understanding of the immune system and tissue development.
Keywords: Granzyme B; cancer; cytotoxicity; extracellular matrix remodeling; immune response; tissue development.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

