A Novel Blood-Based microRNA Diagnostic Model with High Accuracy for Multi-Cancer Early Detection
- PMID: 35326599
- PMCID: PMC8946599
- DOI: 10.3390/cancers14061450
A Novel Blood-Based microRNA Diagnostic Model with High Accuracy for Multi-Cancer Early Detection
Abstract
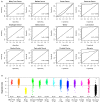
Early detection is critical to reduce cancer deaths as treating early stage cancers is more likely to be successful. However, patients with early stage diseases are often asymptomatic and thus less likely to be diagnosed. Here, we utilized four microarray datasets with a standardized platform to investigate comprehensive microRNA expression profiles from 7536 serum samples. A 4-miRNA diagnostic model was developed from the lung cancer training set (n = 416, 208 lung cancer patients and 208 non-cancer participants). The model showed 99% sensitivity and specificity in the lung cancer validation set (n = 3328, 1358 cancer patients and 1970 non-cancer participants); and the sensitivity remained to be >99% for patients with stage 1 disease. When applied to the additional combined dataset of 3792 participants including 2038 cancer patients across 12 different cancer types and 1754 independent non-cancer controls, the model demonstrated high sensitivities ranging from 83.2 to 100% for biliary tract, bladder, colorectal, esophageal, gastric, glioma, liver, pancreatic, and prostate cancers, and showed reasonable sensitivities of 68.2 and 72.0% for ovarian cancer and sarcoma, respectively, while maintaining 99.3% specificity. Our study provided a proof-of-concept data in demonstrating that the 4-miRNA model has the potential to be developed into a simple, inexpensive and noninvasive blood test for early detection of multiple cancers with high accuracy.
Keywords: blood-based diagnostic model; microRNA; multi-cancer early detection; noninvasive.
Conflict of interest statement
H.H. is a cofounder of miRoncol Diagnostics, a company that seeks to commercialize the diagnostic model.
Figures


References
-
- Noone A.M., Howlader N., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D.R., et al. SEER Cancer Statistics Review, 1975–2015. National Cancer Institute; Rockville, MD, USA: 2018.
LinkOut - more resources
Full Text Sources
Other Literature Sources

