The Thermal Dose of Photothermal Therapy Generates Differential Immunogenicity in Human Neuroblastoma Cells
- PMID: 35326601
- PMCID: PMC8945975
- DOI: 10.3390/cancers14061447
The Thermal Dose of Photothermal Therapy Generates Differential Immunogenicity in Human Neuroblastoma Cells
Abstract
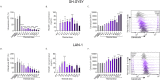
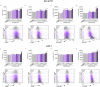
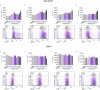
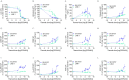
Photothermal therapy (PTT) is an effective method for tumor eradication and has been successfully combined with immunotherapy. However, besides its cytotoxic effects, little is known about the effect of the PTT thermal dose on the immunogenicity of treated tumor cells. Therefore, we administered a range of thermal doses using Prussian blue nanoparticle-based photothermal therapy (PBNP-PTT) and assessed their effects on tumor cell death and concomitant immunogenicity correlates in two human neuroblastoma cell lines: SH-SY5Y (MYCN-non-amplified) and LAN-1 (MYCN-amplified). PBNP-PTT generated thermal dose-dependent tumor cell killing and immunogenic cell death (ICD) in both tumor lines in vitro. However, the effect of the thermal dose on ICD and the expression of costimulatory molecules, immune checkpoint molecules, major histocompatibility complexes, an NK cell-activating ligand, and a neuroblastoma-associated antigen were significantly more pronounced in SH-SY5Y cells compared with LAN-1 cells, consistent with the high-risk phenotype of LAN-1 cells. In functional co-culture studies in vitro, T cells exhibited significantly higher cytotoxicity toward SH-SY5Y cells relative to LAN-1 cells at equivalent thermal doses. This preliminary report suggests the importance of moving past the traditional focus of using PTT solely for tumor eradication to one that considers the immunogenic effects of PTT thermal dose to facilitate its success in cancer immunotherapy.
Keywords: MYCN amplification; Prussian blue nanoparticles; immunogenic cell death; immunogenicity; nanoimmunotherapy; neuroblastoma; photothermal therapy; thermal dose.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Engineered tumor-specific T cells using immunostimulatory photothermal nanoparticles.Cytotherapy. 2023 Jul;25(7):718-727. doi: 10.1016/j.jcyt.2023.03.014. Cytotherapy. 2023. PMID: 37278683 Free PMC article.
-
An Engineered Prussian Blue Nanoparticles-based Nanoimmunotherapy Elicits Robust and Persistent Immunological Memory in a TH-MYCN Neuroblastoma Model.Adv Nanobiomed Res. 2021 Aug;1(8):2100021. doi: 10.1002/anbr.202100021. Epub 2021 Mar 13. Adv Nanobiomed Res. 2021. PMID: 34435194 Free PMC article.
-
Photothermal Therapy Generates a Thermal Window of Immunogenic Cell Death in Neuroblastoma.Small. 2018 May;14(20):e1800678. doi: 10.1002/smll.201800678. Epub 2018 Apr 17. Small. 2018. PMID: 29665282
-
Photothermal therapies to improve immune checkpoint blockade for cancer.Int J Hyperthermia. 2020 Dec;37(3):34-49. doi: 10.1080/02656736.2020.1797190. Int J Hyperthermia. 2020. PMID: 33426992 Free PMC article. Review.
-
How Did Conventional Nanoparticle-Mediated Photothermal Therapy Become "Hot" in Combination with Cancer Immunotherapy?Cancers (Basel). 2022 Apr 18;14(8):2044. doi: 10.3390/cancers14082044. Cancers (Basel). 2022. PMID: 35454950 Free PMC article. Review.
Cited by
-
Engineering Versatile Nanomedicines for Ultrasonic Tumor Immunotherapy.Adv Sci (Weinh). 2024 Jan;11(3):e2305392. doi: 10.1002/advs.202305392. Epub 2023 Dec 2. Adv Sci (Weinh). 2024. PMID: 38041509 Free PMC article. Review.
-
Engineered tumor-specific T cells using immunostimulatory photothermal nanoparticles.Cytotherapy. 2023 Jul;25(7):718-727. doi: 10.1016/j.jcyt.2023.03.014. Cytotherapy. 2023. PMID: 37278683 Free PMC article.
-
Neuroblastoma-A Review of Combination Immunotherapy.Int J Mol Sci. 2024 Jul 15;25(14):7730. doi: 10.3390/ijms25147730. Int J Mol Sci. 2024. PMID: 39062971 Free PMC article. Review.
-
Immunomodulatory R848-Loaded Anti-PD-L1-Conjugated Reduced Graphene Oxide Quantum Dots for Photothermal Immunotherapy of Glioblastoma.Pharmaceutics. 2024 Aug 13;16(8):1064. doi: 10.3390/pharmaceutics16081064. Pharmaceutics. 2024. PMID: 39204409 Free PMC article.
-
Photothermal therapy co-localized with CD137 agonism improves survival in an SM1 melanoma model without hepatotoxicity.Nanomedicine (Lond). 2024;19(25):2049-2064. doi: 10.1080/17435889.2024.2389770. Epub 2024 Sep 3. Nanomedicine (Lond). 2024. PMID: 39225150 Free PMC article.
References
-
- Hoffman H.A., Chakarbarti L., Dumont M.F., Sandler A.D., Fernandes R. Prussian blue nanoparticles for laser-induced photothermal therapy of tumors. RSC Adv. 2014;4:29729–29734. doi: 10.1039/C4RA05209A. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

