Radiolabeled Antibodies for Cancer Imaging and Therapy
- PMID: 35326605
- PMCID: PMC8946248
- DOI: 10.3390/cancers14061454
Radiolabeled Antibodies for Cancer Imaging and Therapy
Abstract
Radioimmunoconjugates consist of a monoclonal antibody (mAb) linked to a radionuclide. Radioimmunoconjugates as theranostics tools have been in development with success, particularly in hematological malignancies, leading to approval by the US Food and Drug Administration (FDA) for the treatment of non-Hodgkin's lymphoma. Radioimmunotherapy (RIT) allows for reduced toxicity compared to conventional radiation therapy and enhances the efficacy of mAbs. In addition, using radiolabeled mAbs with imaging methods provides critical information on the pharmacokinetics and pharmacodynamics of therapeutic agents with direct relevance to the optimization of the dose and dosing schedule, real-time antigen quantitation, antigen heterogeneity, and dynamic antigen changes. All of these parameters are critical in predicting treatment responses and identifying patients who are most likely to benefit from treatment. Historically, RITs have been less effective in solid tumors; however, several strategies are being investigated to improve their therapeutic index, including targeting patients with minimal disease burden; using pre-targeting strategies, newer radionuclides, and improved labeling techniques; and using combined modalities and locoregional application. This review provides an overview of the radiolabeled intact antibodies currently in clinical use and those in development.
Keywords: radioimmunotherapy; radioisotopes; radiolabeled monoclonal antibodies; theranostics.
Conflict of interest statement
H.K.G has received Institutional support for trials from AbbVie. A.M.S is an inventor of patents relating to mAb806 and has received Institutional support for trials from AbbVie, Astra Zeneca, Merck, Telix, Fusion.
Figures
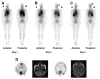
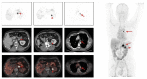
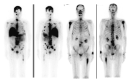
Similar articles
-
Radionuclide therapy of cancer with radiolabeled antibodies.Anticancer Agents Med Chem. 2007 May;7(3):335-43. doi: 10.2174/187152007780618126. Anticancer Agents Med Chem. 2007. PMID: 17504159 Review.
-
Recent preclinical and clinical advances in radioimmunotherapy for non-Hodgkin's lymphoma.Explor Target Antitumor Ther. 2024;5(1):208-224. doi: 10.37349/etat.2024.00213. Epub 2024 Feb 28. Explor Target Antitumor Ther. 2024. PMID: 38464386 Free PMC article. Review.
-
Treatment of non-Hodgkin's lymphoma (NHL) with radiolabeled antibodies (mAbs).Semin Nucl Med. 2005 Jul;35(3):202-11. doi: 10.1053/j.semnuclmed.2005.02.006. Semin Nucl Med. 2005. PMID: 16098294
-
Antibody-guided radiation therapy of cancer.Cancer Metastasis Rev. 2005 Dec;24(4):539-67. doi: 10.1007/s10555-005-6195-z. Cancer Metastasis Rev. 2005. PMID: 16408161 Review.
-
Development of radioimmunotherapy for the treatment of non-Hodgkin's lymphoma.Int J Hematol. 2002 Dec;76(5):401-10. doi: 10.1007/BF02982805. Int J Hematol. 2002. PMID: 12512834 Review.
Cited by
-
Single-Domain Antibodies as Antibody-Drug Conjugates: From Promise to Practice-A Systematic Review.Cancers (Basel). 2024 Jul 27;16(15):2681. doi: 10.3390/cancers16152681. Cancers (Basel). 2024. PMID: 39123409 Free PMC article. Review.
-
The Nitrile Bis-Thiol Bioconjugation Reaction.J Am Chem Soc. 2024 Jan 10;146(1):274-280. doi: 10.1021/jacs.3c08762. Epub 2023 Dec 20. J Am Chem Soc. 2024. PMID: 38124442 Free PMC article.
-
Cancer therapy with antibodies.Nat Rev Cancer. 2024 Jun;24(6):399-426. doi: 10.1038/s41568-024-00690-x. Epub 2024 May 13. Nat Rev Cancer. 2024. PMID: 38740967 Free PMC article. Review.
-
Light-Induced Synthesis and Radiotheranostic Treatment of Gastric Cancer with 161Tb-Labeled Monoclonal Antibodies.JACS Au. 2025 May 22;5(6):2606-2618. doi: 10.1021/jacsau.5c00219. eCollection 2025 Jun 23. JACS Au. 2025. PMID: 40575318 Free PMC article.
-
Radiolabelling and preclinical characterisation of [89Zr]Zr-Df-ATG-101 bispecific to PD-L1/4-1BB.Eur J Nucl Med Mol Imaging. 2024 Sep;51(11):3202-3214. doi: 10.1007/s00259-024-06742-6. Epub 2024 May 11. Eur J Nucl Med Mol Imaging. 2024. PMID: 38730087 Free PMC article.
References
-
- Barbet J., Bardiès M., Bourgeois M., Chatal J.-F., Chérel M., Davodeau F., Faivre-Chauvet A., Gestin J.-F., Kraeber-Bodéré F. Radiolabeled antibodies for cancer imaging and therapy. Antib. Eng. 2012;907:681–697. - PubMed
-
- Harsini S., Rezaei N. Cancer Immunology. Springer; Cham, Switzerland: 2020. Cancer imaging with radiolabeled monoclonal antibodies; pp. 739–760.
Publication types
LinkOut - more resources
Full Text Sources

