Dramatic In Vivo Efficacy of the EZH2-Inhibitor Tazemetostat in PBRM1-Mutated Human Chordoma Xenograft
- PMID: 35326637
- PMCID: PMC8946089
- DOI: 10.3390/cancers14061486
Dramatic In Vivo Efficacy of the EZH2-Inhibitor Tazemetostat in PBRM1-Mutated Human Chordoma Xenograft
Abstract
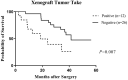
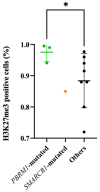
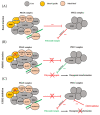
Chordomas are rare neoplasms characterized by a high recurrence rate and a poor long-term prognosis. Considering their chemo-/radio-resistance, alternative treatment strategies are strongly required, but their development is limited by the paucity of relevant preclinical models. Mutations affecting genes of the SWI/SNF complexes are frequently found in chordomas, suggesting a potential therapeutic effect of epigenetic regulators in this pathology. Twelve PDX models were established and characterized on histological and biomolecular features. Patients whose tumors were able to grow into mice had a statistically significant lower progression-free survival than those whose tumors did not grow after in vivo transplantation (p = 0.007). All PDXs maintained the same histopathological features as patients' tumors. Homozygous deletions of CDKN2A/2B (58.3%) and PBRM1 (25%) variants were the most common genomic alterations found. In the tazemetostat treated PDX model harboring a PBRM1 variant, an overall survival of 100% was observed. Our panel of chordoma PDXs represents a useful preclinical tool for both pharmacologic and biological assessments. The first demonstration of a high antitumor activity of tazemetostat in a PDX model harboring a PBRM1 variant supports further evaluation for EZH2-inhibitors in this subgroup of chordomas.
Keywords: EZH2 inhibitor; chordoma; next-generation sequencing; patient-derived xenografts; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
The mutational landscape of skull base and spinal chordomas and the identification of potential prognostic and theranostic biomarkers.J Neurosurg. 2023 Apr 7;139(5):1270-1280. doi: 10.3171/2023.1.JNS222180. Print 2023 Nov 1. J Neurosurg. 2023. PMID: 37029667
-
In vivo efficacy assessment of the CDK4/6 inhibitor palbociclib and the PLK1 inhibitor volasertib in human chordoma xenografts.Front Oncol. 2022 Nov 25;12:960720. doi: 10.3389/fonc.2022.960720. eCollection 2022. Front Oncol. 2022. PMID: 36505864 Free PMC article.
-
Whole genome sequencing of skull-base chordoma reveals genomic alterations associated with recurrence and chordoma-specific survival.Nat Commun. 2021 Feb 3;12(1):757. doi: 10.1038/s41467-021-21026-5. Nat Commun. 2021. PMID: 33536423 Free PMC article.
-
Targeted Therapy for Relapsed/Refractory Follicular Lymphoma: Focus on Clinical Utility of Tazemetostat.Onco Targets Ther. 2022 Feb 27;15:193-199. doi: 10.2147/OTT.S267011. eCollection 2022. Onco Targets Ther. 2022. PMID: 35250278 Free PMC article. Review.
-
[Chordoma].Neurochirurgie. 2014 Jun;60(3):63-140. doi: 10.1016/j.neuchi.2014.02.003. Epub 2014 May 23. Neurochirurgie. 2014. PMID: 24856008 Review. French.
Cited by
-
Cell-Free DNA Extracted from CSF for the Molecular Diagnosis of Pediatric Embryonal Brain Tumors.Cancers (Basel). 2023 Jul 7;15(13):3532. doi: 10.3390/cancers15133532. Cancers (Basel). 2023. PMID: 37444642 Free PMC article.
-
Combinatorial therapies for epigenetic, immunotherapeutic, and genetic targeting of chordoma.J Neurooncol. 2025 Apr;172(2):307-315. doi: 10.1007/s11060-024-04920-y. Epub 2024 Dec 31. J Neurooncol. 2025. PMID: 39738914 Review.
-
Evaluation of Combined Chemotherapy and Genomic-Driven Targeted Therapy in Patient-Derived Xenografts Identifies New Therapeutic Approaches in Squamous Non-Small-Cell Lung Cancer Patients.Cancers (Basel). 2024 Aug 7;16(16):2785. doi: 10.3390/cancers16162785. Cancers (Basel). 2024. PMID: 39199558 Free PMC article.
-
Exploring the therapeutic potential of targeting polycomb repressive complex 2 in lung cancer.Front Oncol. 2023 Oct 16;13:1216289. doi: 10.3389/fonc.2023.1216289. eCollection 2023. Front Oncol. 2023. PMID: 37909018 Free PMC article. Review.
-
Conventional Spinal Chordomas: Investigation of SMARCB1/INI1 Protein Expression, Genetic Alterations in SMARCB1 Gene, and Clinicopathological Features in 89 Patients.Cancers (Basel). 2024 Aug 9;16(16):2808. doi: 10.3390/cancers16162808. Cancers (Basel). 2024. PMID: 39199581 Free PMC article.
References
-
- Stacchiotti S., Gronchi A., Fossati P., Akiyama T., Alapetite C., Baumann M., Blay J.Y., Bolle S., Boriani S., Bruzzi P., et al. Best practices for the management of local-regional recurrent chordoma: A position paper by the Chordoma Global Consensus Group. Ann. Oncol. 2017;28:1230–1242. doi: 10.1093/annonc/mdx054. - DOI - PMC - PubMed
-
- Gill C.M., Fowkes M., Shrivastava R.K. Emerging Therapeutic Targets in Chordomas: A Review of the Literature in the Genomic Era. Neurosurgery [Internet] 2019. [(accessed on 24 March 2020)]. Available online: https://academic.oup.com/neurosurgery/advance-article/doi/10.1093/neuros.... - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

