Molecular Correlates of Venous Thromboembolism (VTE) in Ovarian Cancer
- PMID: 35326647
- PMCID: PMC8946269
- DOI: 10.3390/cancers14061496
Molecular Correlates of Venous Thromboembolism (VTE) in Ovarian Cancer
Abstract
Background: The incidence of venous thromboembolism (VTE) in patients with ovarian cancer is higher than most solid tumors, ranging between 10-30%, and a diagnosis of VTE in this patient population is associated with worse oncologic outcomes. The tumor-specific molecular factors that may lead to the development of VTE are not well understood.
Objectives: The aim of this study was to identify molecular features present in ovarian tumors of patients with VTE compared to those without.
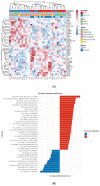
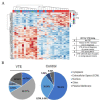
Methods: We performed a multiplatform omics analysis incorporating RNA and DNA sequencing, quantitative proteomics, as well as immune cell profiling of high-grade serous ovarian carcinoma (HGSC) samples from a cohort of 32 patients with or without VTE.
Results: Pathway analyses revealed upregulation of both inflammatory and coagulation pathways in the VTE group. While DNA whole-exome sequencing failed to identify significant coding alterations between the groups, the results of an integrated proteomic and RNA sequencing analysis indicated that there is a relationship between VTE and the expression of platelet-derived growth factor subunit B (PDGFB) and extracellular proteins in tumor cells, namely collagens, that are correlated with the formation of thrombosis.
Conclusions: In this comprehensive analysis of HGSC tumor tissues from patients with and without VTE, we identified markers unique to the VTE group that could contribute to development of thrombosis. Our findings provide additional insights into the molecular alterations underlying the development of VTE in ovarian cancer patients and invite further investigation into potential predictive biomarkers of VTE in ovarian cancer.
Keywords: genetic markers; genomics; ovarian cancer; proteomics; venous thromboembolism.
Conflict of interest statement
A.K. Sood reports consulting for Merck, Kiyatec and GSK, research support from M Trap, and acting as a shareholder for BioPath Holdings. N.D. Fleming reports consulting fees from GSK advisory board. All other authors declare no potential conflict of interest.
Figures


Similar articles
-
Establishment of a mouse model for ovarian cancer-associated venous thromboembolism.Exp Biol Med (Maywood). 2023 Jan;248(1):26-35. doi: 10.1177/15353702221118533. Epub 2022 Aug 29. Exp Biol Med (Maywood). 2023. PMID: 36036485 Free PMC article.
-
PDGFB, a new candidate plasma biomarker for venous thromboembolism: results from the VEREMA affinity proteomics study.Blood. 2016 Dec 8;128(23):e59-e66. doi: 10.1182/blood-2016-05-711846. Epub 2016 Oct 14. Blood. 2016. PMID: 27742707 Clinical Trial.
-
A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS-Cancer-Associated Thrombosis Study.Oncologist. 2017 Oct;22(10):1222-1231. doi: 10.1634/theoncologist.2016-0414. Epub 2017 May 26. Oncologist. 2017. PMID: 28550032 Free PMC article.
-
Incidence and risk factors for postoperative venous thromboembolism in patients with ovarian cancer: Systematic review and meta-analysis.Gynecol Oncol. 2021 Feb;160(2):610-618. doi: 10.1016/j.ygyno.2020.11.010. Epub 2020 Nov 19. Gynecol Oncol. 2021. PMID: 33221022
-
Risk factors for venous thromboembolism in cancer: novel findings from the Vienna Cancer and Thrombosis Study (CATS).Thromb Res. 2014 May;133 Suppl 2:S39-43. doi: 10.1016/S0049-3848(14)50007-2. Thromb Res. 2014. PMID: 24862144 Review.
Cited by
-
Treatment of ovarian cancer: From the past to the new era (Review).Oncol Lett. 2025 Jun 3;30(2):384. doi: 10.3892/ol.2025.15130. eCollection 2025 Aug. Oncol Lett. 2025. PMID: 40535104 Free PMC article. Review.
-
Pancreatic Cancer and Venous Thromboembolism.Int J Mol Sci. 2024 May 23;25(11):5661. doi: 10.3390/ijms25115661. Int J Mol Sci. 2024. PMID: 38891849 Free PMC article. Review.
-
Endothelial Dysfunction Markers in Ovarian Cancer: VTE Risk and Tumour Prognostic Outcomes.Life (Basel). 2024 Dec 9;14(12):1630. doi: 10.3390/life14121630. Life (Basel). 2024. PMID: 39768338 Free PMC article.
-
Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements.Int J Mol Sci. 2024 Feb 3;25(3):1845. doi: 10.3390/ijms25031845. Int J Mol Sci. 2024. PMID: 38339123 Free PMC article. Review.
-
Construction and Validation of a Nomogram to Predict the Postoperative Venous Thromboembolism Risk in Patients with HGSOC.Clin Appl Thromb Hemost. 2024 Jan-Dec;30:10760296241255958. doi: 10.1177/10760296241255958. Clin Appl Thromb Hemost. 2024. PMID: 38767088 Free PMC article.
References
-
- Trousseau A. Clinique Medicale de l’Hotel-Dieu de Paris. Baillière; London, UK: 1865. Phlegmasia alba dolens; pp. 654–712.
-
- Rumbaut R.E., Thiagarajan P. Platelet-Vessel Wall Interactions in Hemostasis and Thrombosis. Morgan & Claypool; San Rafael, CA, USA: 2010. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

