Ion Channel Drugs Suppress Cancer Phenotype in NG108-15 and U87 Cells: Toward Novel Electroceuticals for Glioblastoma
- PMID: 35326650
- PMCID: PMC8946312
- DOI: 10.3390/cancers14061499
Ion Channel Drugs Suppress Cancer Phenotype in NG108-15 and U87 Cells: Toward Novel Electroceuticals for Glioblastoma
Abstract
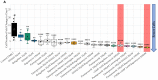
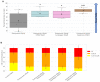
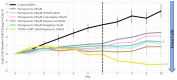
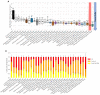
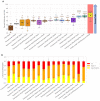
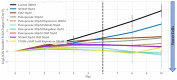
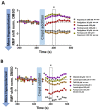
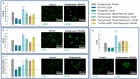
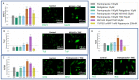
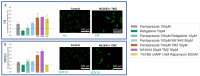
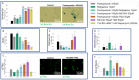
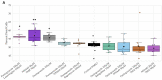
Glioblastoma is a lethal brain cancer that commonly recurs after tumor resection and chemotherapy treatment. Depolarized resting membrane potentials and an acidic intertumoral extracellular pH have been associated with a proliferative state and drug resistance, suggesting that forced hyperpolarization and disruption of proton pumps in the plasma membrane could be a successful strategy for targeting glioblastoma overgrowth. We screened 47 compounds and compound combinations, most of which were ion-modulating, at different concentrations in the NG108-15 rodent neuroblastoma/glioma cell line. A subset of these were tested in the U87 human glioblastoma cell line. A FUCCI cell cycle reporter was stably integrated into both cell lines to monitor proliferation and cell cycle response. Immunocytochemistry, electrophysiology, and a panel of physiological dyes reporting voltage, calcium, and pH were used to characterize responses. The most effective treatments on proliferation in U87 cells were combinations of NS1643 and pantoprazole; retigabine and pantoprazole; and pantoprazole or NS1643 with temozolomide. Marker analysis and physiological dye signatures suggest that exposure to bioelectric drugs significantly reduces proliferation, makes the cells senescent, and promotes differentiation. These results, along with the observed low toxicity in human neurons, show the high efficacy of electroceuticals utilizing combinations of repurposed FDA approved drugs.
Keywords: NG108-15; U89; blockers; cancer; glioblastoma; ion channel; openers.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the experiments, the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures






Similar articles
-
Heterogeneous glioblastoma cell cross-talk promotes phenotype alterations and enhanced drug resistance.Oncotarget. 2015 Dec 1;6(38):40998-1017. doi: 10.18632/oncotarget.5701. Oncotarget. 2015. PMID: 26517510 Free PMC article.
-
In vitro novel combinations of psychotropics and anti-cancer modalities in U87 human glioblastoma cells.Int J Oncol. 2010 Oct;37(4):1043-51. doi: 10.3892/ijo_00000756. Int J Oncol. 2010. PMID: 20811727
-
Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration.J Neurosurg. 2017 May;126(5):1448-1460. doi: 10.3171/2016.1.JNS152077. Epub 2016 Jul 15. J Neurosurg. 2017. PMID: 27419830 Free PMC article.
-
Enhanced anticancer properties of lomustine in conjunction with docosahexaenoic acid in glioblastoma cell lines.J Neurosurg. 2015 Mar;122(3):547-56. doi: 10.3171/2014.10.JNS14759. Epub 2014 Dec 19. J Neurosurg. 2015. PMID: 25526274
-
Alterations of ionic membrane permeabilities in multidrug-resistant neuroblastoma x glioma hybrid cells.J Exp Biol. 1998 Jan;201(Pt 1):21-31. doi: 10.1242/jeb.201.1.21. J Exp Biol. 1998. PMID: 9390933
Cited by
-
Future medicine: from molecular pathways to the collective intelligence of the body.Trends Mol Med. 2023 Sep;29(9):687-710. doi: 10.1016/j.molmed.2023.06.007. Epub 2023 Jul 20. Trends Mol Med. 2023. PMID: 37481382 Free PMC article. Review.
-
Application of Biophysical Techniques to Cellular and Molecular Oncology.Cancers (Basel). 2023 May 26;15(11):2919. doi: 10.3390/cancers15112919. Cancers (Basel). 2023. PMID: 37296882 Free PMC article.
-
Electrical excitability of cancer cells-CELEX model updated.Cancer Metastasis Rev. 2024 Dec;43(4):1579-1591. doi: 10.1007/s10555-024-10195-6. Epub 2024 Jul 8. Cancer Metastasis Rev. 2024. PMID: 38976181 Free PMC article. Review.
-
Information integration during bioelectric regulation of morphogenesis of the embryonic frog brain.iScience. 2023 Nov 4;26(12):108398. doi: 10.1016/j.isci.2023.108398. eCollection 2023 Dec 15. iScience. 2023. PMID: 38034358 Free PMC article.
-
Meeting Review: "National Cancer Institute Conference on Cancer Bioelectricity" September 12, 2024.Bioelectricity. 2025 Mar 18;7(1):94-104. doi: 10.1089/bioe.2024.0049. eCollection 2025 Mar. Bioelectricity. 2025. PMID: 40342939 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

