Divergent Metabolic Effects of Metformin Merge to Enhance Eicosapentaenoic Acid Metabolism and Inhibit Ovarian Cancer In Vivo
- PMID: 35326656
- PMCID: PMC8946838
- DOI: 10.3390/cancers14061504
Divergent Metabolic Effects of Metformin Merge to Enhance Eicosapentaenoic Acid Metabolism and Inhibit Ovarian Cancer In Vivo
Abstract
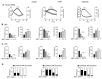
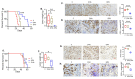
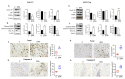
Metformin is being actively repurposed for the treatment of gynecologic malignancies including ovarian cancer. We investigated if metformin induces analogous metabolic changes across ovarian cancer cells. Functional metabolic analysis showed metformin caused an immediate and sustained decrease in oxygen consumption while increasing glycolysis across A2780, C200, and SKOV3ip cell lines. Untargeted metabolomics showed metformin to have differential effects on glycolysis and TCA cycle metabolites, while consistent increased fatty acid oxidation intermediates were observed across the three cell lines. Metabolite set enrichment analysis showed alpha-linolenic/linoleic acid metabolism as being most upregulated. Downstream mediators of the alpha-linolenic/linoleic acid metabolism, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), were abundant in all three cell lines. EPA was more effective in inhibiting SKOV3 and CaOV3 xenografts, which correlated with inhibition of inflammatory markers and indicated a role for EPA-derived specialized pro-resolving mediators such as Resolvin E1. Thus, modulation of the metabolism of omega-3 fatty acids and their anti-inflammatory signaling molecules appears to be one of the common mechanisms of metformin's antitumor activity. The distinct metabolic signature of the tumors may indicate metformin response and aid the preclinical and clinical interpretation of metformin therapy in ovarian and other cancers.
Keywords: DHA; EPA; metabolomics; metformin; omega-3 fatty acids; ovarian cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Lengyel E., Litchfield L.M., Mitra A.K., Nieman K.M., Mukherjee A., Zhang Y., Johnson A., Bradaric M., Lee W., Romero I.L. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am. J. Obstet. Gynecol. 2015;212:479.e1–479.e10. doi: 10.1016/j.ajog.2014.10.026. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

