Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial
- PMID: 35326659
- PMCID: PMC8946662
- DOI: 10.3390/cancers14061510
Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial
Erratum in
-
Correction: Wind et al. Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial. Cancers 2022, 14, 1510.Cancers (Basel). 2023 Feb 27;15(5):1485. doi: 10.3390/cancers15051485. Cancers (Basel). 2023. PMID: 36900424 Free PMC article.
Abstract
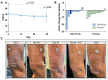
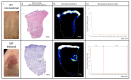
Mycosis fungoides (MF) is a subtype of CTCL with a low incidence and high medical need for novel treatments. The objective of this randomized, placebo-controlled, double-blinded, first-in-human study was to evaluate safety, efficacy, cutaneous and systemic pharmacokinetics (PK) of topical bimiralisib in healthy volunteers (HVs) and MF patients. In this trial, a total of 6 HVs and 19 early-stage MF patients were treated with 2.0% bimiralisib gel and/or placebo. Drug efficacy was assessed by the Composite Assessment of Index Lesion Severity (CAILS) score, supported by objective measuring methods to quantify lesion severity. PK blood samples were collected frequently and cutaneous PK was investigated in skin punch biopsies on the last day of treatment. Local distribution of bimiralisib in HVs showed a mean exposure of 2.54 µg/g in the epidermis. A systemic concentration was observed after application of a target dose of 2 mg/cm2 on 400 cm2, with a mean Cavg of 0.96 ng/mL. Systemic exposure of bimiralisib was reached in all treated MF patients, and normalized plasma concentrations showed a 144% increased exposure compared to HVs, with an observed mean Cavg of 4.49 ng/mL and a mean cutaneous concentration of 5.3 µg/g. No difference in CAILS or objective lesion severity quantification upon 42 days of once-daily treatment was observed in the MF patient group. In general, the treatment was well tolerated in terms of local reactions as well as systemic adverse events. In conclusion, we showed that topical bimiralisib treatment leads to (i) meaningful cutaneous drug levels and (ii) well-tolerated systemic drug exposure in MF patients and (iii) a lack of clinical efficacy, in need of further exploration due to numerous unknown factors, before depreciation of topical bimiralisib as a novel therapeutic drug for CTCLs.
Keywords: CTCL; PI3K/mTOR; PQR309; bimiralisib; mycosis fungoides; pharmacokinetics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Correction: Wind et al. Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial. Cancers 2022, 14, 1510.Cancers (Basel). 2023 Feb 27;15(5):1485. doi: 10.3390/cancers15051485. Cancers (Basel). 2023. PMID: 36900424 Free PMC article.
-
Efficacy and Safety of Topical Hypericin Photodynamic Therapy for Early-Stage Cutaneous T-Cell Lymphoma (Mycosis Fungoides): The FLASH Phase 3 Randomized Clinical Trial.JAMA Dermatol. 2022 Sep 1;158(9):1031-1039. doi: 10.1001/jamadermatol.2022.2749. JAMA Dermatol. 2022. PMID: 35857290 Free PMC article. Clinical Trial.
-
Extracorporeal photophoresis: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(6):1-82. Epub 2006 Mar 1. Ont Health Technol Assess Ser. 2006. PMID: 23074497 Free PMC article.
-
Interventions for mycosis fungoides.Cochrane Database Syst Rev. 2020 Jul 7;7(7):CD008946. doi: 10.1002/14651858.CD008946.pub3. Cochrane Database Syst Rev. 2020. PMID: 32632956 Free PMC article.
-
Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions.J Am Acad Dermatol. 2014 Feb;70(2):223.e1-17; quiz 240-2. doi: 10.1016/j.jaad.2013.08.033. J Am Acad Dermatol. 2014. PMID: 24438970 Review.
Cited by
-
Correction: Wind et al. Topical Bimiralisib Shows Meaningful Cutaneous Drug Levels in Healthy Volunteers and Mycosis Fungoides Patients but No Clinical Activity in a First-in-Human, Randomized Controlled Trial. Cancers 2022, 14, 1510.Cancers (Basel). 2023 Feb 27;15(5):1485. doi: 10.3390/cancers15051485. Cancers (Basel). 2023. PMID: 36900424 Free PMC article.
References
-
- Archier E., Devaux S., Castela E., Gallini A., Aubin A., Le Maître M., Aractingi S., Bachelez H., Cribier B., Joly P., et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2012;26:22–31. doi: 10.1111/j.1468-3083.2012.04520.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

