Impact of Regorafenib on Endothelial Transdifferentiation of Glioblastoma Stem-like Cells
- PMID: 35326702
- PMCID: PMC8946617
- DOI: 10.3390/cancers14061551
Impact of Regorafenib on Endothelial Transdifferentiation of Glioblastoma Stem-like Cells
Abstract
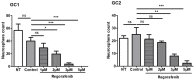
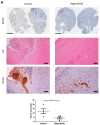
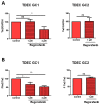
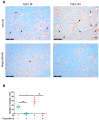
Glioblastomas (GBM) are aggressive brain tumours with a poor prognosis despite heavy therapy that combines surgical resection and radio-chemotherapy. The presence of a subpopulation of GBM stem cells (GSC) contributes to tumour aggressiveness, resistance and recurrence. Moreover, GBM are characterised by abnormal, abundant vascularisation. Previous studies have shown that GSC are directly involved in new vessel formation via their transdifferentiation into tumour-derived endothelial cells (TDEC) and that irradiation (IR) potentiates the pro-angiogenic capacity of TDEC via the Tie2 signalling pathway. We therefore investigated the impact of regorafenib, a multikinase inhibitor with anti-angiogenic and anti-tumourigenic activity, on GSC and TDEC obtained from irradiated GSC (TDEC IR+) or non-irradiated GSC (TDEC). Regorafenib significantly decreases GSC neurosphere formation in vitro and inhibits tumour formation in the orthotopic xenograft model. Regorafenib also inhibits transdifferentiation by decreasing CD31 expression, CD31+ cell count, pseudotube formation in vitro and the formation of functional blood vessels in vivo of TDEC and TDEC IR+. All of these results confirm that regorafenib clearly impacts GSC tumour formation and transdifferentiation and may therefore be a promising therapeutic option in combination with chemo/radiotherapy for the treatment of highly aggressive brain tumours.
Keywords: glioblastoma; glioblastoma stem-like cells; ionising radiation; regorafenib; transdifferentiation; tumour-derived endothelial cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Regorafenib and glioblastoma: a literature review of preclinical studies, molecular mechanisms and clinical effectiveness.Expert Rev Mol Med. 2024 Apr 2;26:e5. doi: 10.1017/erm.2024.8. Expert Rev Mol Med. 2024. PMID: 38563164 Free PMC article. Review.
-
Ionizing radiation induces endothelial transdifferentiation of glioblastoma stem-like cells through the Tie2 signaling pathway.Cell Death Dis. 2019 Oct 28;10(11):816. doi: 10.1038/s41419-019-2055-6. Cell Death Dis. 2019. PMID: 31659157 Free PMC article.
-
Extracellular vesicles produced by irradiated endothelial or Glioblastoma stem cells promote tumor growth and vascularization modulating tumor microenvironment.Cancer Cell Int. 2024 Feb 12;24(1):72. doi: 10.1186/s12935-024-03253-0. Cancer Cell Int. 2024. PMID: 38347567 Free PMC article.
-
BMP9 counteracts the tumorigenic and pro-angiogenic potential of glioblastoma.Cell Death Differ. 2018 Nov;25(10):1808-1822. doi: 10.1038/s41418-018-0149-9. Epub 2018 Jul 5. Cell Death Differ. 2018. PMID: 29977042 Free PMC article.
-
Characteristics of vasculogenic mimicry and tumour to endothelial transdifferentiation in human glioblastoma: a systematic review.BMC Cancer. 2023 Feb 23;23(1):185. doi: 10.1186/s12885-023-10659-y. BMC Cancer. 2023. PMID: 36823554 Free PMC article.
Cited by
-
Glioblastoma-Specific Strategies of Vascularization: Implications in Anti-Angiogenic Therapy Resistance.J Pers Med. 2022 Oct 1;12(10):1625. doi: 10.3390/jpm12101625. J Pers Med. 2022. PMID: 36294763 Free PMC article. Review.
-
Endothelial transdifferentiation of glioma stem cells: a literature review.Acta Neuropathol Commun. 2025 Aug 21;13(1):181. doi: 10.1186/s40478-025-02031-x. Acta Neuropathol Commun. 2025. PMID: 40841988 Free PMC article. Review.
-
Regorafenib and glioblastoma: a literature review of preclinical studies, molecular mechanisms and clinical effectiveness.Expert Rev Mol Med. 2024 Apr 2;26:e5. doi: 10.1017/erm.2024.8. Expert Rev Mol Med. 2024. PMID: 38563164 Free PMC article. Review.
-
Editorial for "Impact of Regorafenib on Endothelial Transdifferentiation of Glioblastoma Stem-like Cells".Cancers (Basel). 2023 Jul 28;15(15):3830. doi: 10.3390/cancers15153830. Cancers (Basel). 2023. PMID: 37568645 Free PMC article.
-
A Review of FDA-Approved Multi-Target Angiogenesis Drugs for Brain Tumor Therapy.Int J Mol Sci. 2025 Feb 28;26(5):2192. doi: 10.3390/ijms26052192. Int J Mol Sci. 2025. PMID: 40076810 Free PMC article. Review.
References
-
- Louis D.N., Ohgaki H., Wiestler O.D., Cavenee W.K. World Health Organization Histological Classifications of Tumours of the Central Nervous System. 4th ed. IARC Press; Lyon, France: 2016. - PubMed
-
- Stupp R., Hegi M.E., Mason W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:8. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

