Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy
- PMID: 35326708
- PMCID: PMC8946148
- DOI: 10.3390/cancers14061555
Emerging Roles for Mammalian Target of Rapamycin (mTOR) Complexes in Bladder Cancer Progression and Therapy
Abstract
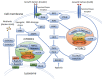
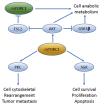
The mammalian target of rapamycin (mTOR) pathway regulates important cellular functions. Aberrant activation of this pathway, either through upstream activation by growth factors, loss of inhibitory controls, or molecular alterations, can enhance cancer growth and progression. Bladder cancer shows high levels of mTOR activity in approximately 70% of urothelial carcinomas, suggesting a key role for this pathway in this cancer. mTOR signaling initiates through upstream activation of phosphatidylinositol 3 kinase (PI3K) and protein kinase B (AKT) and results in activation of either mTOR complex 1 (mTORC1) or mTOR complex 2 (mTORC2). While these complexes share several key protein components, unique differences in their complex composition dramatically alter the function and downstream cellular targets of mTOR activity. While significant work has gone into analysis of molecular alterations of the mTOR pathway in bladder cancer, this has not yielded significant benefit in mTOR-targeted therapy approaches in urothelial carcinoma to date. New discoveries regarding signaling convergence onto mTOR complexes in bladder cancer could yield unique insights the biology and targeting of this aggressive disease. In this review, we highlight the functional significance of mTOR signaling in urothelial carcinoma and its potential impact on future therapy implications.
Keywords: bladder cancer; inhibitor; invasion; mTOR; progression; targeted therapy; urothelial carcinoma.
Conflict of interest statement
In the last 3 years (unrelated to this manuscript), Grivas has received consulting fees from AstraZeneca, Astellas Pharma, Bayer, Bristol Myers Squibb, Clovis Oncology, Dyania Health, EMD Serono, Exelixis, Foundation Medicine, Genentech/Roche, Genzyme, GlaxoSmithKline, Guardant Health, Immunomedics/Gilead, Infinity Pharmaceuticals, Janssen, Merck & Co., Mirati Therapeutics, Pfizer, QED Therapeutics, Regeneron Pharmaceuticals, Seattle Genetics, 4D Pharma PLC, UroGen. His institution has received grants from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm, EMD Serono, GlaxoSmithKline, Immunomedics/Gilead, Kure It Cancer Research, Merck & Co., Mirati Therapeutics, Pfizer, QED Therapeutics, G1 Therapeutics. The other authors declare no relevant conflict of interest.
Figures


Similar articles
-
A comprehensive immunohistochemical and molecular approach to the PI3K/AKT/mTOR (phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene/mammalian target of rapamycin) pathway in bladder urothelial carcinoma.BJU Int. 2012 Dec;110(11 Pt C):E1237-48. doi: 10.1111/j.1464-410X.2012.11569.x. Epub 2012 Oct 29. BJU Int. 2012. PMID: 23107319
-
Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2.PLoS Biol. 2009 Feb 10;7(2):e38. doi: 10.1371/journal.pbio.1000038. PLoS Biol. 2009. PMID: 19209957 Free PMC article.
-
Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship.Adv Biol Regul. 2019 May;72:51-62. doi: 10.1016/j.jbior.2019.03.003. Epub 2019 Apr 11. Adv Biol Regul. 2019. PMID: 31010692 Review.
-
Disentangling the signaling pathways of mTOR complexes, mTORC1 and mTORC2, as a therapeutic target in glioblastoma.Adv Biol Regul. 2022 Jan;83:100854. doi: 10.1016/j.jbior.2021.100854. Epub 2021 Dec 6. Adv Biol Regul. 2022. PMID: 34996736 Review.
-
Mammalian target of rapamycin inhibition in hepatocellular carcinoma.World J Hepatol. 2014 Nov 27;6(11):776-82. doi: 10.4254/wjh.v6.i11.776. World J Hepatol. 2014. PMID: 25429315 Free PMC article. Review.
Cited by
-
Anti-tumor effect of AZD8055 against bladder cancer and bladder cancer-associated macrophages.Heliyon. 2023 Mar 6;9(3):e14272. doi: 10.1016/j.heliyon.2023.e14272. eCollection 2023 Mar. Heliyon. 2023. PMID: 36938467 Free PMC article.
-
Lactate-related metabolic reprogramming and immune regulation in colorectal cancer.Front Endocrinol (Lausanne). 2023 Jan 26;13:1089918. doi: 10.3389/fendo.2022.1089918. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36778600 Free PMC article. Review.
-
The role of E3 ubiquitin ligases and deubiquitinases in bladder cancer development and immunotherapy.Front Immunol. 2023 May 5;14:1202633. doi: 10.3389/fimmu.2023.1202633. eCollection 2023. Front Immunol. 2023. PMID: 37215134 Free PMC article. Review.
-
Epigenetic Biomarkers as a New Diagnostic Tool in Bladder Cancer-From Early Detection to Prognosis.J Clin Med. 2024 Nov 26;13(23):7159. doi: 10.3390/jcm13237159. J Clin Med. 2024. PMID: 39685620 Free PMC article. Review.
-
The Potential of Congo Red Supplied Aggregates of Multitargeted Tyrosine Kinase Inhibitor (Sorafenib, BAY-43-9006) in Enhancing Therapeutic Impact on Bladder Cancer.Int J Mol Sci. 2023 Dec 23;25(1):269. doi: 10.3390/ijms25010269. Int J Mol Sci. 2023. PMID: 38203437 Free PMC article.
References
-
- Mock H., Humphrey P.A., Ulbright T.M., Reuter V.E. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. Volume 8 International Agency for Research on Cancer; Lyon, France: 2016.
-
- Witjes J.A., Bruins H.M., Cathomas R., Comperat E.M., Cowan N.C., Gakis G., Hernandez V., Linares Espinos E., Lorch A., Neuzillet Y., et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021;79:82–104. doi: 10.1016/j.eururo.2020.03.055. - DOI - PubMed
-
- Daneshmand S., Svatek R., Singh P., Woldu S. Bladder Neoplasms: Muscle Invasive Bladder Cancer. [(accessed on 8 December 2021)]. Available online: https://university.auanet.org/core/oncology-adult/bladder-neoplasms-musc....
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

