A Brief Overview of Adaptive Designs for Phase I Cancer Trials
- PMID: 35326715
- PMCID: PMC8946506
- DOI: 10.3390/cancers14061566
A Brief Overview of Adaptive Designs for Phase I Cancer Trials
Abstract
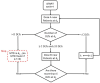
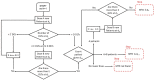
Phase I studies are used to estimate the dose-toxicity profile of the drugs and to select appropriate doses for successive studies. However, literature on statistical methods used for phase I studies are extensive. The objective of this review is to provide a concise summary of existing and emerging techniques for selecting dosages that are appropriate for phase I cancer trials. Many advanced statistical studies have proposed novel and robust methods for adaptive designs that have shown significant advantages over conventional dose finding methods. An increasing number of phase I cancer trials use adaptive designs, particularly during the early phases of the study. In this review, we described nonparametric and algorithm-based designs such as traditional 3 + 3, accelerated titration, Bayesian algorithm-based design, up-and-down design, and isotonic design. In addition, we also described parametric model-based designs such as continual reassessment method, escalation with overdose control, and Bayesian decision theoretic and optimal design. Ongoing studies have been continuously focusing on improving and refining the existing models as well as developing newer methods. This study would help readers to assimilate core concepts and compare different phase I statistical methods under one banner. Nevertheless, other evolving methods require future reviews.
Keywords: adaptive design; algorithm-based designs; cancer clinical trial; dose-limiting toxicity; maximum tolerated dose; nonparametric designs; parametric model-based designs; phase I trial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Systematic comparison of the statistical operating characteristics of various Phase I oncology designs.Contemp Clin Trials Commun. 2016 Nov 24;5:34-48. doi: 10.1016/j.conctc.2016.11.006. eCollection 2017 Mar. Contemp Clin Trials Commun. 2016. PMID: 29740620 Free PMC article.
-
A comparison of phase I dose-finding designs in clinical trials with monotonicity assumption violation.Clin Trials. 2020 Oct;17(5):522-534. doi: 10.1177/1740774520932130. Epub 2020 Jul 7. Clin Trials. 2020. PMID: 32631095
-
Comparative review of novel model-assisted designs for phase I clinical trials.Stat Med. 2018 Jun 30;37(14):2208-2222. doi: 10.1002/sim.7674. Epub 2018 Apr 22. Stat Med. 2018. PMID: 29682777 Review.
-
Modeling adverse event counts in phase I clinical trials of a cytotoxic agent.Clin Trials. 2018 Aug;15(4):386-397. doi: 10.1177/1740774518772309. Epub 2018 May 19. Clin Trials. 2018. PMID: 29779418 Free PMC article.
-
Adaptive dose-finding studies: a review of model-guided phase I clinical trials.J Clin Oncol. 2014 Aug 10;32(23):2505-11. doi: 10.1200/JCO.2013.54.6051. Epub 2014 Jun 30. J Clin Oncol. 2014. PMID: 24982451 Free PMC article. Review.
Cited by
-
Translational Challenges and Prospective Solutions in the Implementation of Biomimetic Delivery Systems.Pharmaceutics. 2023 Nov 14;15(11):2623. doi: 10.3390/pharmaceutics15112623. Pharmaceutics. 2023. PMID: 38004601 Free PMC article. Review.
-
SPIRIT-DEFINE explanation and elaboration: recommendations for enhancing quality and impact of early phase dose-finding clinical trials protocols.EClinicalMedicine. 2025 Jan 10;79:102988. doi: 10.1016/j.eclinm.2024.102988. eCollection 2025 Jan. EClinicalMedicine. 2025. PMID: 39877554 Free PMC article.
-
CONSORT-DEFINE explanation and elaboration: recommendations for enhancing reporting quality and impact of early phase dose-finding clinical trials.EClinicalMedicine. 2025 Jan 10;79:102987. doi: 10.1016/j.eclinm.2024.102987. eCollection 2025 Jan. EClinicalMedicine. 2025. PMID: 39877553 Free PMC article.
-
Enhancing reporting quality and impact of early phase dose-finding clinical trials: CONSORT Dose-finding Extension (CONSORT-DEFINE) guidance.BMJ. 2023 Oct 20;383:e076387. doi: 10.1136/bmj-2023-076387. BMJ. 2023. PMID: 37863501 Free PMC article.
-
Safety and Tolerability of an Antimalarial Herbal Remedy in Healthy Volunteers: An Open-Label, Single-Arm, Dose-Escalation Study on Maytenus senegalensis in Tanzania.Trop Med Infect Dis. 2022 Nov 25;7(12):396. doi: 10.3390/tropicalmed7120396. Trop Med Infect Dis. 2022. PMID: 36548651 Free PMC article.
References
-
- Rukhsar L., Bangyal W.H., Ali Khan M.S., Ibrahim A., Asri A., Nisar K., Rawat D.B. Analyzing RNA-Seq Gene Expression Data Using Deep Learning Approaches for Cancer Classification. Appl. Sci. 2022;12:1850. doi: 10.3390/app12041850. - DOI
-
- Nagendran M., Chen Y., Lovejoy C.A., Gordon A.C., Komorowski M., Harvey H., Topol E.J., Ioannidis J.P., Collins G.S., Maruthappu M. Artificial intelligence versus clinicians: Systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;25:368. doi: 10.1136/bmj.m689. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources