A Brief Overview of Adaptive Designs for Phase I Cancer Trials
- PMID: 35326715
- PMCID: PMC8946506
- DOI: 10.3390/cancers14061566
A Brief Overview of Adaptive Designs for Phase I Cancer Trials
Abstract
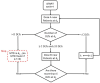
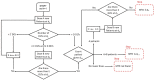
Phase I studies are used to estimate the dose-toxicity profile of the drugs and to select appropriate doses for successive studies. However, literature on statistical methods used for phase I studies are extensive. The objective of this review is to provide a concise summary of existing and emerging techniques for selecting dosages that are appropriate for phase I cancer trials. Many advanced statistical studies have proposed novel and robust methods for adaptive designs that have shown significant advantages over conventional dose finding methods. An increasing number of phase I cancer trials use adaptive designs, particularly during the early phases of the study. In this review, we described nonparametric and algorithm-based designs such as traditional 3 + 3, accelerated titration, Bayesian algorithm-based design, up-and-down design, and isotonic design. In addition, we also described parametric model-based designs such as continual reassessment method, escalation with overdose control, and Bayesian decision theoretic and optimal design. Ongoing studies have been continuously focusing on improving and refining the existing models as well as developing newer methods. This study would help readers to assimilate core concepts and compare different phase I statistical methods under one banner. Nevertheless, other evolving methods require future reviews.
Keywords: adaptive design; algorithm-based designs; cancer clinical trial; dose-limiting toxicity; maximum tolerated dose; nonparametric designs; parametric model-based designs; phase I trial.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rukhsar L., Bangyal W.H., Ali Khan M.S., Ibrahim A., Asri A., Nisar K., Rawat D.B. Analyzing RNA-Seq Gene Expression Data Using Deep Learning Approaches for Cancer Classification. Appl. Sci. 2022;12:1850. doi: 10.3390/app12041850. - DOI
-
- Nagendran M., Chen Y., Lovejoy C.A., Gordon A.C., Komorowski M., Harvey H., Topol E.J., Ioannidis J.P., Collins G.S., Maruthappu M. Artificial intelligence versus clinicians: Systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020;25:368. doi: 10.1136/bmj.m689. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources