MRI Response Assessment in Glioblastoma Patients Treated with Dendritic-Cell-Based Immunotherapy
- PMID: 35326730
- PMCID: PMC8946797
- DOI: 10.3390/cancers14061579
MRI Response Assessment in Glioblastoma Patients Treated with Dendritic-Cell-Based Immunotherapy
Abstract
Introduction: In this post hoc analysis we compared various response-assessment criteria in newly diagnosed glioblastoma (GB) patients treated with tumor lysate-charged autologous dendritic cells (Audencel) and determined the differences in prediction of progression-free survival (PFS) and overall survival (OS). Methods: 76 patients enrolled in a multicenter phase II trial receiving standard of care (SOC, n = 40) or SOC + Audencel vaccine (n = 36) were included. MRI scans were evaluated using MacDonald, RANO, Vol-RANO, mRANO, Vol-mRANO and iRANO criteria. Tumor volumes (T1 contrast-enhancing as well as T2/FLAIR volumes) were calculated by semiautomatic segmentation. The Kruskal-Wallis-test was used to detect differences in PFS among the assessment criteria; for correlation analysis the Spearman test was used. Results: There was a significant difference in median PFS between mRANO (8.6 months) and Vol-mRANO (8.6 months) compared to MacDonald (4.0 months), RANO (4.2 months) and Vol-RANO (5.4 months). For the vaccination arm, median PFS by iRANO was 6.2 months. There was no difference in PFS between SOC and SOC + Audencel. The best correlation between PFS/OS was detected for mRANO (r = 0.65) and Vol-mRANO (r = 0.69, each p < 0.001). A total of 16/76 patients developed a pure T2/FLAIR progressing disease, and 4/36 patients treated with Audencel developed pseudoprogression. Conclusion: When comparing different response-assessment criteria in GB patients treated with dendritic cell-based immunotherapy, the best correlation between PFS and OS was observed for mRANO and Vol-mRANO. Interestingly, iRANO was not superior for predicting OS in patients treated with Audencel.
Keywords: glioblastoma; iRANO; immunotherapy; mRANO; radiologic response criteria; volumetric measurements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
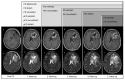
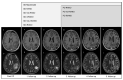
References
-
- Wöhrer A., Waldhör T., Heinzl H., Hackl M., Feichtinger J., Gruber-Mösenbacher U., Kiefer A., Maier H., Motz R., Reiner-Concin A., et al. The Austrian Brain Tumour Registry: A cooperative way to establish a population-based brain tumour registry. J. Neurooncol. 2009;95:401–411. doi: 10.1007/s11060-009-9938-9. - DOI - PubMed
-
- Stupp R., Taillibert S., Kanner A.A., Kesari S., Steinberg D.M., Toms S.A., Taylor L.P., Lieberman F., Silvani A., Fink K.L., et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015;314:2535–2543. doi: 10.1001/jama.2015.16669. - DOI - PubMed
-
- Herrlinger U., Tzaridis T., Mack F., Steinbach J.P., Schlegel U., Sabel M., Hau P., Kortmann R.D., Krex D., Grauer O., et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet. 2019;393:678–688. doi: 10.1016/S0140-6736(18)31791-4. - DOI - PubMed
LinkOut - more resources
Full Text Sources

