The Barcelona Predictive Model of Clinically Significant Prostate Cancer
- PMID: 35326740
- PMCID: PMC8946272
- DOI: 10.3390/cancers14061589
The Barcelona Predictive Model of Clinically Significant Prostate Cancer
Abstract
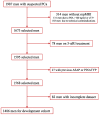
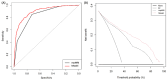
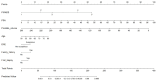
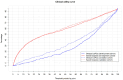
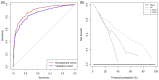
A new and externally validated MRI-PM for csPCa was developed in the metropolitan area of Barcelona, and a web-RC designed with the new option of selecting the csPCa probability threshold. The development cohort comprised 1486 men scheduled to undergo a 3-tesla multiparametric MRI (mpMRI) and guided and/or systematic biopsies in one academic institution of Barcelona. The external validation cohort comprised 946 men in whom the same diagnostic approach was carried out as in the development cohort, in two other academic institutions of the same metropolitan area. CsPCa was detected in 36.9% of men in the development cohort and 40.8% in the external validation cohort (p = 0.054). The area under the curve of mpMRI increased from 0.842 to 0.897 in the developed MRI-PM (p < 0.001), and from 0.743 to 0.858 in the external validation cohort (p < 0.001). A selected 15% threshold avoided 40.1% of prostate biopsies and missed 5.4% of the 36.9% csPCa detected in the development cohort. In men with PI-RADS <3, 4.3% would be biopsied and 32.3% of all existing 4.2% of csPCa would be detected. In men with PI-RADS 3, 62% of prostate biopsies would be avoided and 28% of all existing 12.4% of csPCa would be undetected. In men with PI-RADS 4, 4% of prostate biopsies would be avoided and 0.6% of all existing 43.1% of csPCa would be undetected. In men with PI-RADS 5, 0.6% of prostate biopsies would be avoided and none of the existing 42.0% of csPCa would be undetected. The Barcelona MRI-PM presented good performance on the overall population; however, its clinical usefulness varied regarding the PI-RADS category. The selection of csPCa probability thresholds in the designed RC may facilitate external validation and outperformance of MRI-PMs in specific PI-RADS categories.
Keywords: clinically significant prostate cancer; magnetic resonance imaging; predictive model; risk calculator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mottet N., van den Bergh R.C.N., Briesrs E., Briers E., Van den Broeck T., Cumberbatch M.G., De Santis M., Fanti S., Fossati N., Gandaglia G., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021;79:243–262. doi: 10.1016/j.eururo.2020.09.042. - DOI - PubMed
-
- Ahmed H.U., El-Shater Bosaily A., Brown L.C., Gabe R., Kaplan R., Parmar M.K., Collaco-Moraes Y., Ward K., Hindley R.G., Freeman A., et al. Diagnostic accuracy of multi-parametric MRI and TRUSbiopsy in prostate cancer (PROMIS): A paired validating confirmatory study. Lancet. 2017;389:815–822. doi: 10.1016/S0140-6736(16)32401-1. - DOI - PubMed
-
- Van Poppel H., Roobol M.J., Chapple C.R., Catto J.W.F., N’Dow J., Sønksen J., Stenzl A., Wirth M. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur. Urol. 2021;80:703–711. doi: 10.1016/j.eururo.2021.07.024. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

