In Vitro Screening of a 1280 FDA-Approved Drugs Library against Multidrug-Resistant and Extensively Drug-Resistant Bacteria
- PMID: 35326755
- PMCID: PMC8944690
- DOI: 10.3390/antibiotics11030291
In Vitro Screening of a 1280 FDA-Approved Drugs Library against Multidrug-Resistant and Extensively Drug-Resistant Bacteria
Abstract
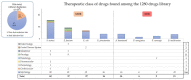
Alternative strategies against multidrug-resistant (MDR) bacterial infections are suggested to clinicians, such as drug repurposing, which uses rapidly available and marketed drugs. We gathered a collection of MDR bacteria from our hospital and performed a phenotypic high-throughput screening with a 1280 FDA-approved drug library. We used two Gram positive (Enterococcus faecium P5014 and Staphylococcus aureus P1943) and six Gram negative (Acinetobacter baumannii P1887, Klebsiella pneumoniae P9495, Pseudomonas aeruginosa P6540, Burkholderia multivorans P6539, Pandoraea nosoerga P8103, and Escherichia coli DSM105182 as the reference and control strain). The selected MDR strain panel carried resistance genes or displayed phenotypic resistance to last-line therapies such as carbapenems, vancomycin, or colistin. A total of 107 compounds from nine therapeutic classes inhibited >90% of the growth of the selected Gram negative and Gram positive bacteria at a drug concentration set at 10 µmol/L, and 7.5% were anticancer drugs. The common hit was the antiseptic chlorhexidine. The activity of niclosamide, carmofur, and auranofin was found against the selected methicillin-resistant S. aureus. Zidovudine was effective against colistin-resistant E. coli and carbapenem-resistant K. pneumoniae. Trifluridine, an antiviral, was effective against E. faecium. Deferoxamine mesylate inhibited the growth of XDR P. nosoerga. Drug repurposing by an in vitro screening of a drug library is a promising approach to identify effective drugs for specific bacteria.
Keywords: alternative strategy; antibiotic combination; drug repurposing; extensively-drug resistant bacteria; multidrug-resistant bacteria; old drugs.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
Repurposing Non-Antibiotic Drugs Auranofin and Pentamidine in Combination to Combat Multidrug-Resistant Gram-Negative Bacteria.Int J Antimicrob Agents. 2022 May;59(5):106582. doi: 10.1016/j.ijantimicag.2022.106582. Epub 2022 Apr 1. Int J Antimicrob Agents. 2022. PMID: 35378227
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
[Microbiological profiles of pathogens causing nosocomial bacteremia in 2011, 2013 and 2016].Sheng Wu Gong Cheng Xue Bao. 2018 Aug 25;34(8):1205-1217. doi: 10.13345/j.cjb.180192. Sheng Wu Gong Cheng Xue Bao. 2018. PMID: 30152206 Chinese.
-
Molecular characterization of multidrug-resistant ESKAPEE pathogens from clinical samples in Chonburi, Thailand (2017-2018).BMC Infect Dis. 2022 Aug 17;22(1):695. doi: 10.1186/s12879-022-07678-8. BMC Infect Dis. 2022. PMID: 35978294 Free PMC article.
-
Multidrug-Resistant and Virulent Organisms Trauma Infections: Trauma Infectious Disease Outcomes Study Initiative.Mil Med. 2022 May 4;187(Suppl 2):42-51. doi: 10.1093/milmed/usab131. Mil Med. 2022. PMID: 35512375 Free PMC article. Review.
Cited by
-
Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria.Microorganisms. 2022 Jun 16;10(6):1239. doi: 10.3390/microorganisms10061239. Microorganisms. 2022. PMID: 35744757 Free PMC article. Review.
-
Repurposing a drug to punish carbapenem-resistant Acinetobacter baumannii.Proc Natl Acad Sci U S A. 2025 Jun 17;122(24):e2423650122. doi: 10.1073/pnas.2423650122. Epub 2025 Jun 10. Proc Natl Acad Sci U S A. 2025. PMID: 40493197
-
A metagenomic library cloning strategy that promotes high-level expression of captured genes to enable efficient functional screening.Cell Chem Biol. 2023 Dec 21;30(12):1680-1691.e6. doi: 10.1016/j.chembiol.2023.10.001. Epub 2023 Oct 27. Cell Chem Biol. 2023. PMID: 37898120 Free PMC article.
-
Impact of Growth Conditions on High-Throughput Identification of Repurposing Drugs for Pseudomonas aeruginosa Cystic Fibrosis Lung Infections.Antibiotics (Basel). 2024 Jul 12;13(7):642. doi: 10.3390/antibiotics13070642. Antibiotics (Basel). 2024. PMID: 39061324 Free PMC article.
-
High-Throughput Screening of Natural Product and Synthetic Molecule Libraries for Antibacterial Drug Discovery.Metabolites. 2023 May 2;13(5):625. doi: 10.3390/metabo13050625. Metabolites. 2023. PMID: 37233666 Free PMC article. Review.
References
-
- Musso M., Mosti S., Gualano G., Mencarini P., Urso R., Ghirga P., Rianda A., Del Nonno F., Goletti D., Palmieri F. Hepatitis C virus infection: A challenge in the complex management of two cases of multidrug-resistant tuberculosis. BMC Infect. Dis. 2019;19:882. doi: 10.1186/s12879-019-4494-1. - DOI - PMC - PubMed
-
- Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. - DOI - PubMed
-
- Kadri S.S., Adjemian J., Lai Y.L., Spaulding A.B., Ricotta E., Prevots D.R., Palmore T.N., Rhee C., Klompas M., Dekker J.P., et al. Difficult-to-Treat Resistance in Gram-negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-line Agents. Clin. Infect. Dis. 2018;67:1803–1814. doi: 10.1093/cid/ciy378. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

