Recent Trends in Prostate Biopsy Complication Rates and the Role of Aztreonam in Periprocedural Antimicrobial Prophylaxis-A Nationwide Population-Based Study from Korea
- PMID: 35326775
- PMCID: PMC8944457
- DOI: 10.3390/antibiotics11030312
Recent Trends in Prostate Biopsy Complication Rates and the Role of Aztreonam in Periprocedural Antimicrobial Prophylaxis-A Nationwide Population-Based Study from Korea
Abstract
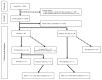
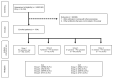
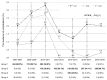
An increase in the rate of complications after prostate biopsy (PB) due to increased antibiotic-resistant bacteria is a global issue. We report the safety of aztreonam as a prophylactic antibiotic in patients undergoing PB. We investigated the complication rates according to several antibiotic regimens, including aztreonam. We hypothesized that PB complications increased following a rise in antibiotic-resistant bacteria. We examined the annual rates of complications among patients in our hospital (clinical cohort) and the Korea Health Insurance Review and Assessment Service (HIRA) cohort. Data regarding complications, hospitalization, emergency room (ER) visits, and febrile urinary tract infections occurring within 2 weeks after PB were recorded. The rate of complications was significantly lower in patients who received oral quinolone and intravenous aztreonam than in those who received oral quinolone. The complication rates did not increase throughout the study period. Additionally, 1754 patients from the HIRA cohort were included. The rates of complications, hospitalizations, and ER visits did not increase among these patients. Oral quinolone combined with intravenous aztreonam reduced the rate of febrile complications compared to quinolone alone and was safe to use after PB. Therefore, we recommend intravenous aztreonam with oral quinolone as a prophylactic antibiotic regimen before PB.
Keywords: aztreonam; prophylactic antibiotic; prostate biopsy; susceptibility.
Conflict of interest statement
The authors declare no conflict of interest. The funding had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Clinical importance of the antibiotic regimen in transrectal ultrasound-guided biopsy: quinolone versus cephalosporin.BMC Urol. 2016 Aug 24;16(1):51. doi: 10.1186/s12894-016-0169-z. BMC Urol. 2016. PMID: 27557527 Free PMC article.
-
Febrile urinary tract infection after prostate biopsy and quinolone resistance.Korean J Urol. 2014 Oct;55(10):660-4. doi: 10.4111/kju.2014.55.10.660. Epub 2014 Oct 10. Korean J Urol. 2014. PMID: 25324949 Free PMC article.
-
Cost-effectiveness of standard vs intensive antibiotic regimens for transrectal ultrasonography (TRUS)-guided prostate biopsy prophylaxis.BJU Int. 2012 Jul;110(2 Pt 2):E86-91. doi: 10.1111/j.1464-410X.2011.10768.x. Epub 2011 Nov 24. BJU Int. 2012. PMID: 22115356
-
The role of aztreonam in treatment of complicated urinary tract infections in children.Pediatr Infect Dis J. 1989 Sep;8(9 Suppl):S113-6; discussion S128-32. doi: 10.1097/00006454-198909001-00005. Pediatr Infect Dis J. 1989. PMID: 2682509 Review.
-
Prevention of infectious complications after prostate biopsy procedure.Int J Urol. 2017 Jul;24(7):486-492. doi: 10.1111/iju.13369. Epub 2017 May 27. Int J Urol. 2017. PMID: 28556409 Review.
Cited by
-
Prostate biopsy in patients without rectal access: a systematic review and proportional meta-analysis.Cent European J Urol. 2025;78(1):14-22. doi: 10.5173/ceju.2024.0097. Epub 2025 Jan 24. Cent European J Urol. 2025. PMID: 40371426 Free PMC article. Review.
References
-
- Nam R.K., Saskin R., Lee Y., Liu Y., Law C., Klotz L.H., Loblaw D.A., Trachtenberg J., Stanimirovic A., Simor A.E., et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J. Urol. 2013;189:S12–S18. doi: 10.1016/j.juro.2012.11.015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

