Effects of Lysine N-ζ-Methylation in Ultrashort Tetrabasic Lipopeptides (UTBLPs) on the Potentiation of Rifampicin, Novobiocin, and Niclosamide in Gram-Negative Bacteria
- PMID: 35326798
- PMCID: PMC8963254
- DOI: 10.3390/antibiotics11030335
Effects of Lysine N-ζ-Methylation in Ultrashort Tetrabasic Lipopeptides (UTBLPs) on the Potentiation of Rifampicin, Novobiocin, and Niclosamide in Gram-Negative Bacteria
Abstract
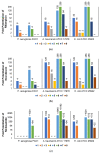
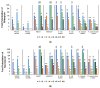
Outer membrane (OM) drug impermeability typically associated with a molecular weight above 600 Da and high hydrophobicity prevents accumulation of many antibiotics in Gram-negative bacteria (GNB). Previous studies have shown that ultrashort tetrabasic lipopeptides (UTBLPs) containing multiple lysine residues potentiate Gram-positive bacteria (GPB)-selective antibiotics in GNB by enhancing OM permeability. However, there is no available information on how N-substitution at the ζ-position of lysine in UTBLPs affects antibiotic potentiation in GNB. To study these effects, we prepared a series of branched and linear UTBLPs that differ in the degree of N-ζ-methylation and studied their potentiating effects with GPB-selective antibiotics including rifampicin, novobiocin, niclosamide, and chloramphenicol against wild-type and multidrug-resistant GNB isolates. Our results show that increasing N-ζ-methylation reduces or abolishes the potentiating effects of UTBLPs with rifampicin, novobiocin, and niclosamide against GNB. No trend was observed with chloramphenicol that is largely affected by efflux. We were unable to observe a correlation between the strength of the antibiotic potentiating effect to the increase in fluorescence in the 1-N-phenylnaphthylamine (NPN) OM permeability assay suggesting that other factors besides OM permeability of NPN play a role in antibiotic potentiation. In conclusion, our study has elucidated crucial structure-activity relationships for the optimization of polybasic antibiotic potentiators in GNB.
Keywords: Acinetobacter baumannii; Escherichia coli; Pseudomonas aeruginosa; antibiotic adjuvant; antibiotic potentiator; niclosamide; novobiocin; outer membrane permeabilizer; rifampicin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Dilipid Ultrashort Tetrabasic Peptidomimetics Potentiate Novobiocin and Rifampicin Against Multidrug-Resistant Gram-Negative Bacteria.ACS Infect Dis. 2020 Jun 12;6(6):1413-1426. doi: 10.1021/acsinfecdis.0c00017. Epub 2020 May 7. ACS Infect Dis. 2020. PMID: 32357292
-
Dioctanoyl Ultrashort Tetrabasic β-Peptides Sensitize Multidrug-Resistant Gram-Negative Bacteria to Novobiocin and Rifampicin.Front Microbiol. 2021 Dec 23;12:803309. doi: 10.3389/fmicb.2021.803309. eCollection 2021. Front Microbiol. 2021. PMID: 35003035 Free PMC article.
-
Amphiphilic tribasic galactosamines potentiate rifampicin in Gram-negative bacteria at low Mg++/Ca++concentrations.Bioorg Med Chem Lett. 2024 Jan 1;97:129371. doi: 10.1016/j.bmcl.2023.129371. Epub 2023 Jun 8. Bioorg Med Chem Lett. 2024. PMID: 37301521
-
New potentiators of ineffective antibiotics: Targeting the Gram-negative outer membrane to overcome intrinsic resistance.Curr Opin Chem Biol. 2022 Feb;66:102099. doi: 10.1016/j.cbpa.2021.102099. Epub 2021 Nov 19. Curr Opin Chem Biol. 2022. PMID: 34808425 Review.
-
Multidrug-resistant bacteria: overcoming antibiotic permeability barriers of gram-negative bacteria.Ann Med. 2001 Apr;33(3):167-71. doi: 10.3109/07853890109002073. Ann Med. 2001. PMID: 11370769 Review.
Cited by
-
Lipopeptide adjuvants for antibiotics and vaccines: the future step in the fight against multidrug-resistant and extensively drug-resistant pathogens.Explor Drug Sci. 2024;2:203-233. doi: 10.37349/eds.2024.00043. Epub 2024 Apr 29. Explor Drug Sci. 2024. PMID: 40842601 Free PMC article.
-
Tackling the outer membrane: facilitating compound entry into Gram-negative bacterial pathogens.NPJ Antimicrob Resist. 2023 Dec 20;1(1):17. doi: 10.1038/s44259-023-00016-1. NPJ Antimicrob Resist. 2023. PMID: 39843585 Free PMC article. Review.
-
Antibiotic Adjuvants: A Versatile Approach to Combat Antibiotic Resistance.ACS Omega. 2023 Mar 14;8(12):10757-10783. doi: 10.1021/acsomega.3c00312. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008128 Free PMC article. Review.
-
Quaternary Ammonium Salts of Cationic Lipopeptides with Lysine Residues - Synthesis, Antimicrobial, Hemolytic and Cytotoxic Activities.Probiotics Antimicrob Proteins. 2023 Dec;15(6):1465-1483. doi: 10.1007/s12602-023-10161-8. Epub 2023 Sep 29. Probiotics Antimicrob Proteins. 2023. PMID: 37770629 Free PMC article.
-
Synergy by Perturbing the Gram-Negative Outer Membrane: Opening the Door for Gram-Positive Specific Antibiotics.ACS Infect Dis. 2022 Sep 9;8(9):1731-1757. doi: 10.1021/acsinfecdis.2c00193. Epub 2022 Aug 10. ACS Infect Dis. 2022. PMID: 35946799 Free PMC article. Review.
References
-
- Antibiotics Currently in Global Clinical Development. [(accessed on 18 January 2022)]. Available online: https://www.pewtrusts.org/en/research-and-analysis/data-visualizations/2....
-
- O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance; London, UK: 2016.
Grants and funding
LinkOut - more resources
Full Text Sources

