Tobramycin Blood Levels after Local Antibiotic Treatment of Bone and Soft Tissue Infection
- PMID: 35326799
- PMCID: PMC8944707
- DOI: 10.3390/antibiotics11030336
Tobramycin Blood Levels after Local Antibiotic Treatment of Bone and Soft Tissue Infection
Abstract
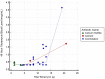
Local antibiotic delivery using different carriers plays an important role in both infection prophylaxis and treatment. Besides dead space management, these carriers have the advantage of providing a high concentration of local antibiotics with a lower risk of systemic toxicity. Few studies have reported on systemic toxicity associated with antibiotic-impregnated carriers. The present study investigates the systemic tobramycin concentration at 24, 48 and 72 h postoperatively after using tobramycin-loaded polymethyl methacrylate (PMMA) and calcium sulfate (CS) as local antibiotic carriers. Additionally, this work assesses the renal function postoperatively for indications of acute kidney injury (AKI). Fifty-two patients were treated in 58 procedures with tobramycin and vancomycin-loaded PMMA, CS, or both. All systemic tobramycin levels were <2 mcg/mL at 72 h, and the resulting rate of AKI was 12% (7/58). In conclusion, local tobramycin antibiotic delivery using PMMA, CS, or both remains a safe and effective modality in the treatment of osteomyelitis as long as the surgeon is aware of its possible nephrotoxic effect.
Keywords: PMMA; acute kidney injury; calcium sulfate; creatinine; safety; tobramycin; toxicity.
Conflict of interest statement
J.D.C. is a consultant for Bonesupport, Orthofix, Smith & Nephew, and Zimmer Biomet, receives fellowship support from Biocomposites, is on the MicroGenDX Advisory Board, and her spouse receives royalties from the University of Florida. The remaining authors declare no conflict of interest. The following organizations supported the institution of C.D.P., A.H.E., M.A., M.G.G., and J.D.C.: DePuy Synthes, Integra LifeSciences, NuVasive Specialized Orthopedics, Orthofix, OrthoPediatrics, Paragon 28, Pega Medical, Smith & Nephew, Stryker, Treace Medical Concepts, and WishBone Medical Inc. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Buchholz H.W., Gartmann H.D. Infection prevention and surgical management of deep insidious infection in total endoprosthesis. [(accessed on 21 January 2022)];Chirurg. 1972 43:446–453. (In German) Available online: https://pubmed.ncbi.nlm.nih.gov/5084870/ - PubMed
LinkOut - more resources
Full Text Sources

