Influence of Sub-Inhibitory Dosage of Cefotaxime on Multidrug Resistant Staphylococcus haemolyticus Isolated from Sick Neonatal Care Unit
- PMID: 35326823
- PMCID: PMC8944431
- DOI: 10.3390/antibiotics11030360
Influence of Sub-Inhibitory Dosage of Cefotaxime on Multidrug Resistant Staphylococcus haemolyticus Isolated from Sick Neonatal Care Unit
Abstract
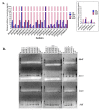
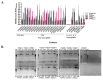
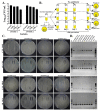
Staphylococcus haemolyticus has emerged to be a frequently encountered late-onset sepsis pathogen among newborn infants. Critical care of neonates involves substantial usage of antibiotics and these pathogens are often exposed to sub-optimal doses of antibiotics which can augment maintenance of selection determinants and a range of physiological effects, prime among them being biofilm formation. Therefore, in this study, the outcome of a sub-inhibitory dosage of a commonly prescribed third-generation antibiotic, cefotaxime (CTX), on multidrug resistant (MDR) S. haemolyticus, was investigated. A total of 19 CTX-resistant, MDR and 5 CTX-susceptible strains isolated from neonates were included. Biofilm-forming abilities of S. haemolyticus isolates in the presence of sub-optimal CTX (30 μg/mL) were determined by crystal violet assays and extracellular DNA (eDNA) quantitation. CTX was found to significantly enhance biofilm production among the non-susceptible isolates (p-valueWilcoxintest—0.000008) with an increase in eDNA levels (p-valueWilcoxintest—0.000004). Further, in the absence of antibiotic selection in vitro, populations of MDR isolates, JNM56C1 and JNM60C2 remained antibiotic non-susceptible after >500 generations of growth. These findings demonstrate that sub-optimal concentration of CTX induces biofilm formation and short-term non-exposure to antibiotics does not alter non-susceptibility among S. haemolyticus isolates under the tested conditions.
Keywords: Staphylococcus haemolyticus; biofilms; cefotaxime; neonates; short-term evolution; sub-MIC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evaluation of the antimicrobial function of Ginkgo biloba exocarp extract against clinical bacteria and its effect on Staphylococcus haemolyticus by disrupting biofilms.J Ethnopharmacol. 2022 Nov 15;298:115602. doi: 10.1016/j.jep.2022.115602. Epub 2022 Aug 24. J Ethnopharmacol. 2022. PMID: 36030030
-
Methicillin-Resistant Staphylococcus haemolyticus Displaying Reduced Susceptibility to Vancomycin and High Biofilm-Forming Ability.Infect Disord Drug Targets. 2021;21(7):e160921191517. doi: 10.2174/1871526521666210217151807. Infect Disord Drug Targets. 2021. PMID: 33596813
-
Sphygmomanometers and thermometers as potential fomites of Staphylococcus haemolyticus: biofilm formation in the presence of antibiotics.Mem Inst Oswaldo Cruz. 2017 Mar;112(3):188-195. doi: 10.1590/0074-02760160381. Epub 2017 Feb 16. Mem Inst Oswaldo Cruz. 2017. PMID: 28225903 Free PMC article.
-
[Phenotypic evaluation of hydrophobicity and the ability to produce biofilm in coagulase-negative staphylococci isolated from infected very-low-birthweight newborns].Med Dosw Mikrobiol. 2013;65(3):149-59. Med Dosw Mikrobiol. 2013. PMID: 24432554 Polish.
-
Clinical Infections, Antibiotic Resistance, and Pathogenesis of Staphylococcus haemolyticus.Microorganisms. 2022 May 31;10(6):1130. doi: 10.3390/microorganisms10061130. Microorganisms. 2022. PMID: 35744647 Free PMC article. Review.
Cited by
-
Genomic and phenotypic characterization of multidrug-resistant Staphylococcus haemolyticus isolated from burn patients in Chongqing, southwestern China.Microbiol Spectr. 2025 Jun 3;13(6):e0257724. doi: 10.1128/spectrum.02577-24. Epub 2025 Apr 17. Microbiol Spectr. 2025. PMID: 40243352 Free PMC article.
References
-
- Zaha D.C., Zdrinca M.M., Vesa C.M., Daina L.G., Daina M.C. A six-year evaluation of sepsis in neonates. Rom. Biotechnol. Lett. 2020;25:1892–1898. doi: 10.25083/rbl/25.5/1892.1898. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

