Antimicrobial Resistance, Biofilm Formation, and Virulence Genes in Enterococcus Species from Small Backyard Chicken Flocks
- PMID: 35326843
- PMCID: PMC8944505
- DOI: 10.3390/antibiotics11030380
Antimicrobial Resistance, Biofilm Formation, and Virulence Genes in Enterococcus Species from Small Backyard Chicken Flocks
Abstract
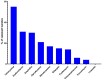
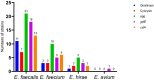
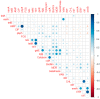
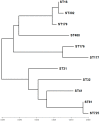
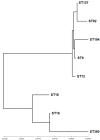
Backyard birds are small flocks that are more common in developing countries. They are used for poultry meat and egg production. However, they are also implicated in the maintenance and transmission of several zoonotic diseases, including multidrug-resistant bacteria. Enterococci are one of the most common zoonotic bacteria. They colonize numerous body sites and cause a wide range of serious nosocomial infections in humans. Therefore, the objective of the present study was to investigate the diversity in Enterococcus spp. in healthy birds and to determine the occurrence of multidrug resistance (MDR), multi-locus sequence types, and virulence genes and biofilm formation. From March 2019 to December 2020, cloacal swabs were collected from 15 healthy backyard broiler flocks. A total of 90 enterococci strains were recovered and classified according to the 16S rRNA sequence into Enterococcus faecalis (50%); Enterococcus faecium (33.33%), Enterococcus hirae (13.33%), and Enterococcus avium (3.33%). The isolates exhibited high resistance to tetracycline (55.6%), erythromycin (31.1%), and ampicillin (30%). However, all of the isolates were susceptible to linezolid. Multidrug resistance (MDR) was identified in 30 (33.3%) isolates. The enterococci AMR-associated genes ermB, ermA, tetM, tetL, vanA, cat, and pbp5 were identified in 24 (26.6%), 11 (12.2%), 39 (43.3%), 34 (37.7%), 1 (1.1%), 4 (4.4%), and 23 (25.5%) isolates, respectively. Of the 90 enterococci, 21 (23.3%), 27 (30%), and 36 (40%) isolates showed the presence of cylA, gelE, and agg virulence-associated genes, respectively. Seventy-three (81.1%) isolates exhibited biofilm formation. A statistically significant correlation was obtained for biofilm formation versus the MAR index and MDR. Multi-locus sequence typing (MLST) identified eleven and eight different STs for E. faecalis and E. faecium, respectively. Seven different rep-family plasmid genes (rep1-2, rep3, rep5-6, rep9, and rep11) were detected in the MDR enterococci. Two-thirds (20/30; 66.6%) of the enterococci were positive for one or two rep-families. In conclusion, the results show that healthy backyard chickens could act as a reservoir for MDR and virulent Enterococcus spp. Thus, an effective antimicrobial stewardship program and further studies using a One Health approach are required to investigate the role of backyard chickens as vectors for AMR transmission to humans.
Keywords: Enterococcus; antimicrobial resistance; antimicrobial-resistance genes; backyard chickens; multidrug resistance; virulence genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ammerlaan H.S., Harbarth S., Buiting A.G., Crook D.W., Fitzpatrick F., Hanberger H., Herwaldt L.A., van Keulen P.H., Kluytmans J.A., Kola A., et al. Secular trends in nosocomial bloodstream infections: Antibiotic-resistant bacteria increase the total burden of infection. Clin. Infect. Dis. 2013;56:798–805. doi: 10.1093/cid/cis1006. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

