Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance
- PMID: 35326853
- PMCID: PMC8944833
- DOI: 10.3390/antibiotics11030390
Nanozybiotics: Nanozyme-Based Antibacterials against Bacterial Resistance
Abstract
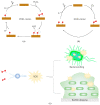
Infectious diseases caused by bacteria represent a global threat to human health. However, due to the abuse of antibiotics, drug-resistant bacteria have evolved rapidly and led to the failure of antibiotics treatment. Alternative antimicrobial strategies different to traditional antibiotics are urgently needed. Enzyme-based antibacterials (Enzybiotics) have gradually attracted interest owing to their advantages including high specificity, rapid mode-of-action, no resistance development, etc. However, due to their low stability, potential immunogenicity, and high cost of natural enzymes, enzybiotics have limitations in practical antibacterial therapy. In recent years, many nanomaterials with enzyme-like activities (Nanozymes) have been discovered as a new generation of artificial enzymes and perform catalytic antibacterial effects against bacterial resistance. To highlight the progress in this field of nanozyme-based antibacterials (Nanozybiotics), this review discussed the antibacterial mechanism of action of nanozybiotics with a comparison with enzybiotics. We propose that nanozybiotics may bear promising applications in antibacterial therapy, due to their high stability, rapid bacterial killing, biofilm elimination, and low cost.
Keywords: bacterial resistance; biofilm; enzybiotics; nanozybiotics; nanozymes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Nanozybiotics: Advancing Antimicrobial Strategies Through Biomimetic Mechanisms.Adv Mater. 2024 Aug;36(33):e2403362. doi: 10.1002/adma.202403362. Epub 2024 Jun 26. Adv Mater. 2024. PMID: 38874860 Review.
-
Nanozymes and their biomolecular conjugates as next-generation antibacterial agents: A comprehensive review.Int J Biol Macromol. 2024 Oct;278(Pt 1):134582. doi: 10.1016/j.ijbiomac.2024.134582. Epub 2024 Aug 8. Int J Biol Macromol. 2024. PMID: 39122068 Review.
-
Progress in antibacterial applications of nanozymes.Front Chem. 2024 Sep 23;12:1478273. doi: 10.3389/fchem.2024.1478273. eCollection 2024. Front Chem. 2024. PMID: 39376729 Free PMC article. Review.
-
Catalytic antimicrobial therapy using nanozymes.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022 Mar;14(2):e1769. doi: 10.1002/wnan.1769. Epub 2021 Dec 22. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2022. PMID: 34939348 Review.
-
Enzybiotics: Enzyme-Based Antibacterials as Therapeutics.Adv Exp Med Biol. 2019;1148:233-253. doi: 10.1007/978-981-13-7709-9_11. Adv Exp Med Biol. 2019. PMID: 31482502 Review.
Cited by
-
Bioconjugation of Serratiopeptidase with Titanium Oxide Nanoparticles: Improving Stability and Antibacterial Properties.J Funct Biomater. 2024 Oct 7;15(10):300. doi: 10.3390/jfb15100300. J Funct Biomater. 2024. PMID: 39452598 Free PMC article.
-
Metal and Metal Oxide Nanomaterials for Fighting Planktonic Bacteria and Biofilms: A Review Emphasizing on Mechanistic Aspects.Int J Mol Sci. 2022 Sep 26;23(19):11348. doi: 10.3390/ijms231911348. Int J Mol Sci. 2022. PMID: 36232647 Free PMC article. Review.
-
Pt-Ru bimetallic nanoclusters with peroxidase-like activity for antibacterial therapy.PLoS One. 2024 May 21;19(5):e0301358. doi: 10.1371/journal.pone.0301358. eCollection 2024. PLoS One. 2024. PMID: 38771804 Free PMC article.
-
Biosimilars Targeting Pathogens: A Comprehensive Review of Their Role in Bacterial, Fungal, Parasitic, and Viral Infections.Pharmaceutics. 2025 Apr 28;17(5):581. doi: 10.3390/pharmaceutics17050581. Pharmaceutics. 2025. PMID: 40430873 Free PMC article. Review.
-
Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections.Antibiotics (Basel). 2024 Jan 25;13(2):121. doi: 10.3390/antibiotics13020121. Antibiotics (Basel). 2024. PMID: 38391507 Free PMC article. Review.
References
-
- Jernigan J.A., Hatfield K.M., Wolford H., Nelson R.E., Olubajo B., Reddy S.C., McCarthy N., Paul P., McDonald L.C., Kallen A., et al. Multidrug-resistant bacterial infections in us hospitalized patients, 2012–2017. N. Engl. J. Med. 2020;382:1309–1319. doi: 10.1056/NEJMoa1914433. - DOI - PMC - PubMed
-
- Bacalum M., Radu M. Cationic Antimicrobial Peptides Cytotoxicity on Mammalian Cells: An Analysis Using Therapeutic Index Integrative Concept. Int. J. Pept. Res. Ther. 2015;21:47–55. doi: 10.1007/s10989-014-9430-z. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

