Studies on the Reactions of Biapenem with VIM Metallo β-Lactamases and the Serine β-Lactamase KPC-2
- PMID: 35326858
- PMCID: PMC8944426
- DOI: 10.3390/antibiotics11030396
Studies on the Reactions of Biapenem with VIM Metallo β-Lactamases and the Serine β-Lactamase KPC-2
Abstract
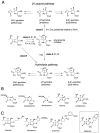
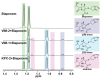
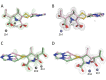
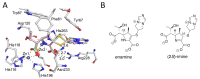
Carbapenems are important antibacterials and are both substrates and inhibitors of some β-lactamases. We report studies on the reaction of the unusual carbapenem biapenem, with the subclass B1 metallo-β-lactamases VIM-1 and VIM-2 and the class A serine-β-lactamase KPC-2. X-ray diffraction studies with VIM-2 crystals treated with biapenem reveal the opening of the β-lactam ring to form a mixture of the (2S)-imine and enamine complexed at the active site. NMR studies on the reactions of biapenem with VIM-1, VIM-2, and KPC-2 reveal the formation of hydrolysed enamine and (2R)- and (2S)-imine products. The combined results support the proposal that SBL/MBL-mediated carbapenem hydrolysis results in a mixture of tautomerizing enamine and (2R)- and (2S)-imine products, with the thermodynamically favoured (2S)-imine being the major observed species over a relatively long-time scale. The results suggest that prolonging the lifetimes of β-lactamase carbapenem complexes by optimising tautomerisation of the nascently formed enamine to the (2R)-imine and likely more stable (2S)-imine tautomer is of interest in developing improved carbapenems.
Keywords: antimicrobial resistance; biapenem; carbapenems; metallo-β-lactamases; serine-β-lactamases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Grants and funding
- EP/L016044/1/MRC_/Medical Research Council/United Kingdom
- BB/M011224/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 106244/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- BB/S50676X//BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/T016035/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

