Impact of COVID-19 Pandemic Declaration on New Oncology Trial Commencements: An Interrupted Time Series with Segmented Regression Analysis
- PMID: 35326967
- PMCID: PMC8953517
- DOI: 10.3390/healthcare10030489
Impact of COVID-19 Pandemic Declaration on New Oncology Trial Commencements: An Interrupted Time Series with Segmented Regression Analysis
Abstract
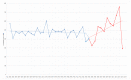
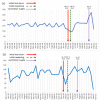
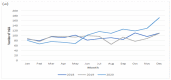
This study aimed to assess the trend in oncology trial commencements registered on ClinicalTrials.gov and to evaluate the contributing factors by comparing the trends in the pre- and post-COVID-19 pandemic era. The ClinicalTrials.gov database was searched to identify oncology study trials starting from 1 January 2018 to 28 February 2021. Data on the variables of start/complete date, phase, status, funding source, center, country and study type were extracted. According to the time point of the COVID-19 pandemic declaration by the World Health Organization (WHO), March 2020, we analyzed the extracted data, including interrupted time series (ITS) analysis and multivariable regression analysis. We identified 18,561 new oncology trials during the study period. A total of 5678 oncology trials in the prepandemic period and 6134 in the postpandemic period were included in the comparative analysis. The year 2020 had the most newly launched trials (32.3%), and the majority of trials were planned to be conducted for longer than two years (70.3%). The results of ITS show the trend in the commencement of oncology trials was significantly increased after the pandemic declaration (coefficient = 27.99; 95% CI = 19.27 to 36.71). Drug intervention trials were the largest contributor to the increased trial number compared to different interventions, such as trials of devices or procedures (OR = 1.14; 95% CI = 1.03 to 1.26, OR = 1.09; 95% CI = 0.91 to 1.29, and OR = 1.12; 95% CI = 0.96 to 1.31, respectively), whereas the United Kingdom was the highest contributor to the number of decreased trials (OR = 0.67; 95% CI = 0.51 to 0.89 p = 0.01) in the postpandemic era. The interruption in oncology trial initiation was diminished shortly after the COVID-19 pandemic declaration, which was influenced by several factors, such as interventions or national responses. Based on the current outcomes, appropriate strategies for developing oncology trials can be planned to mitigate the impact of future crises on oncology trials.
Keywords: COVID-19; clinicaltrial.gov; oncology; pandemic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Impact of the COVID-19 Pandemic on Non-COVID-19 Clinical Trials.J Cardiovasc Dev Dis. 2022 Jan 10;9(1):19. doi: 10.3390/jcdd9010019. J Cardiovasc Dev Dis. 2022. PMID: 35050229 Free PMC article.
-
Evaluation of Oncology Trial Results Reporting Over a 10-Year Period.JAMA Netw Open. 2021 May 3;4(5):e2110438. doi: 10.1001/jamanetworkopen.2021.10438. JAMA Netw Open. 2021. PMID: 34028549 Free PMC article.
-
The COVID-19 pandemic and new clinical trial activations.Trials. 2021 Apr 8;22(1):260. doi: 10.1186/s13063-021-05219-3. Trials. 2021. PMID: 33832534 Free PMC article.
-
Trends of Phase I Clinical Trials in the Latest Ten Years across Five European Countries.Int J Environ Res Public Health. 2022 Oct 28;19(21):14023. doi: 10.3390/ijerph192114023. Int J Environ Res Public Health. 2022. PMID: 36360902 Free PMC article.
-
Trends in the Incidence of New-Onset Anorexia Nervosa and Atypical Anorexia Nervosa Among Youth During the COVID-19 Pandemic in Canada.JAMA Netw Open. 2021 Dec 1;4(12):e2137395. doi: 10.1001/jamanetworkopen.2021.37395. JAMA Netw Open. 2021. PMID: 34874405 Free PMC article.
References
-
- World Health Organization COVID-19 Weekly Epidemiological Update. Feb 20, 2022. [(accessed on 20 February 2022)]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
LinkOut - more resources
Full Text Sources

