Cryopreservation of Testicular Tissue from Adult Red-Rumped Agoutis (Dasyprocta leporina Linnaeus, 1758)
- PMID: 35327135
- PMCID: PMC8944822
- DOI: 10.3390/ani12060738
Cryopreservation of Testicular Tissue from Adult Red-Rumped Agoutis (Dasyprocta leporina Linnaeus, 1758)
Abstract
This study measured the effects of different freezing techniques and permeating cryoprotectants on the preservation of testicular tissues from adult red-rumped agoutis. Tissue biopsies (3.0 mm3) from five individuals were allocated to different experimental groups: control (non-cryopreserved); slow freezing (SF), solid-surface vitrification (SSV), and conventional vitrification (CV). Each method used dimethyl sulfoxide (DMSO), ethylene glycol (EG), or a DMSO + EG combination. Morphology, viability, mitochondrial activity, and proliferative potential were assessed in fresh and frozen tissue samples. Testicular morphology was better using SSV with a combination of DMSO and EG. Across the different cryopreservation approaches, as well as cryoprotectant combinations, cell viability was comparable. Regarding mitochondrial activity, DMSO + EG/SSV or CV, and DMSO + EG/CV were similar to the EG/SF group, which was the best group that provided values similar to fresh control groups. Adequate preservation of the proliferative potential of spermatogonia, Leydig cells, and Sertoli cells was obtained using SSV with DMSO + EG. Overall, the use of SSV with DMSO + EG was the best protocol for the preservation of testicular tissues from adult red-rumped agoutis.
Keywords: biobanking; permeating cryoprotectants; testicular tissue; vitrification; wild rodents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
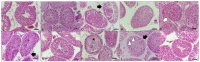
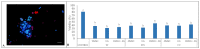
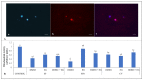
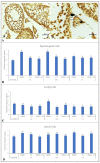
Similar articles
-
Influence of freezing techniques and glycerol-based cryoprotectant combinations on the survival of testicular tissues from adult collared peccaries.Theriogenology. 2021 Jun;167:111-119. doi: 10.1016/j.theriogenology.2021.03.013. Epub 2021 Mar 26. Theriogenology. 2021. PMID: 33813051
-
Comparison of different intracellular cryoprotectants on the solid surface vitrification of red-rumped agouti (Dasyprocta Leporina Lichtenstein, 1823) ovarian tissue.Reprod Domest Anim. 2020 Feb;55(2):154-161. doi: 10.1111/rda.13600. Epub 2019 Dec 24. Reprod Domest Anim. 2020. PMID: 31804747
-
Combination of intracellular cryoprotectants preserves the structure and the cells proliferative capacity potential of adult collared peccary testicular tissue subjected to solid surface vitrification.Cryobiology. 2019 Dec;91:53-60. doi: 10.1016/j.cryobiol.2019.10.199. Epub 2019 Oct 31. Cryobiology. 2019. PMID: 31678072
-
Evaluation of Different Cryoprotectant Combinations in Testicular Vitrification in Dogs.Reprod Domest Anim. 2025 Jun;60(6):e70081. doi: 10.1111/rda.70081. Reprod Domest Anim. 2025. PMID: 40445065 Free PMC article.
-
Solid Surface Vitrification Is Better than Slow Freezing for the Long-Term Preservation of Testicular Fragments from Prepubertal Collared Peccaries (Pecari tajacu Linnaeus, 1758).Animals (Basel). 2025 May 20;15(10):1488. doi: 10.3390/ani15101488. Animals (Basel). 2025. PMID: 40427365 Free PMC article.
Cited by
-
Testicular cryopreservation: From technical aspects to practical applications.Histol Histopathol. 2025 Jul;40(7):967-978. doi: 10.14670/HH-18-869. Epub 2025 Jan 2. Histol Histopathol. 2025. PMID: 39810720 Review.
-
Gonadal tissue preservation technologies and culture offer opportunities to bridge knowledge between wildlife and humans.F S Rep. 2025 Apr 15;6(Suppl 1):50-54. doi: 10.1016/j.xfre.2025.01.009. eCollection 2025 Apr. F S Rep. 2025. PMID: 40487320 Free PMC article.
-
Biobanking and use of gonadal tissues - a promising strategy for conserving wildlife from the Caatinga biome.Anim Reprod. 2023 Feb 13;19(4):e20220135. doi: 10.1590/1984-3143-AR2022-0135. eCollection 2022. Anim Reprod. 2023. PMID: 36819484 Free PMC article.
-
DNA integrity and viability of testicular cells from diverse wild species after slow freezing or vitrification.Front Vet Sci. 2023 Jan 16;9:1114695. doi: 10.3389/fvets.2022.1114695. eCollection 2022. Front Vet Sci. 2023. PMID: 36727036 Free PMC article.
-
Recent Progress of Induced Spermatogenesis In Vitro.Int J Mol Sci. 2024 Aug 5;25(15):8524. doi: 10.3390/ijms25158524. Int J Mol Sci. 2024. PMID: 39126092 Free PMC article. Review.
References
-
- Brown-Uddenberg R.C., Garcia G.W., Baptiste Q.S., Counand T., Adogwa A.O., Sampson T. The Agouti (Dasyprocta leporina, D. aguti) Booklet and Producers Manual. 1st ed. GWG Publications; St. Augustine, Trinidad and Tobago: 2004.
-
- Galetti M., Donatti C.I., Steffler C., Genini J., Bovendorp R.S., Fleury M. The role of seed mass on the caching decision by agoutis, Dasyprocta leporina (Rodentia: Agoutidae) Zoologia. 2010;27:472–476. doi: 10.1590/S1984-46702010000300022. - DOI
-
- Mittelman P., Kreischer C., Pires A.S., Fernandez F.A.S. Agouti reintroduction recovers seed dispersal of a large-seeded tropical tree. Biotropica. 2020;52:766–774. doi: 10.1111/btp.12788. - DOI
-
- Schipper J., Emmons L., McCarthy T. The IUCN Red List of Threatened Species. IUCN; Gland, Switzerland: 2016. Dasyprocta ruatanica; p. e.T6287A22198054.
Grants and funding
LinkOut - more resources
Full Text Sources

