Proteomic Analysis of Intracellular and Membrane-Associated Fractions of Canine (Canis lupus familiaris) Epididymal Spermatozoa and Sperm Structure Separation
- PMID: 35327169
- PMCID: PMC8944539
- DOI: 10.3390/ani12060772
Proteomic Analysis of Intracellular and Membrane-Associated Fractions of Canine (Canis lupus familiaris) Epididymal Spermatozoa and Sperm Structure Separation
Abstract
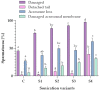
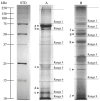
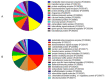
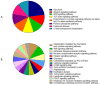
This study was provided for proteomic analysis of intracellular and membrane-associated fractions of canine (Canis lupus familiaris) epididymal spermatozoa and additionally to find optimal sonication parameters for the epididymal sperm morphological structure separation and sperm protein isolation. Sperm samples were collected from 15 dogs. Sperm protein fractions: intracellular (SIPs) and membrane-associated (SMAPs) were isolated. After sonication, sperm morphology was evaluated using Spermac Stain™. The sperm protein fractions were analyzed using gel electrophoresis (SDS-PAGE) and nanoliquid chromatography coupled to quadrupole time-of-flight mass spectrometry (NanoLC-Q-TOF/MS). UniProt database-supported identification resulted in 42 proteins identified in the SIPs and 153 proteins in the SMAPs. Differentially abundant proteins (DAPs) were found in SIPs and SMAPs. Based on a gene ontology analysis, the dominant molecular functions of SIPs were catalytic activity (50%) and binding (28%). Hydrolase activity (33%) and transferase activity (21%) functions were dominant for SMAPs. Bioinformatic analysis of SIPs and SMAPs showed their participation in important metabolic pathways in epididymal sperm, which may suggest their potential as sperm quality biomarkers. The use of sonication 150 W, 10 min, may be recommended for the separation of dog epididymal sperm heads, tails, acrosomes and the protein isolation.
Keywords: canine; epididymal spermatozoa; proteomic; semen quality; sonication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Age-Dependent Variations in Functional Quality and Proteomic Characteristics of Canine (Canis lupus familiaris) Epididymal Spermatozoa.Int J Mol Sci. 2022 Aug 15;23(16):9143. doi: 10.3390/ijms23169143. Int J Mol Sci. 2022. PMID: 36012418 Free PMC article.
-
Proteome Profiling of Canine Epididymal Fluid: In Search of Protein Markers of Epididymal Sperm Motility.Int J Mol Sci. 2023 Sep 30;24(19):14790. doi: 10.3390/ijms241914790. Int J Mol Sci. 2023. PMID: 37834239 Free PMC article.
-
Equine seminal plasma and sperm membrane: Functional proteomic assessment.Theriogenology. 2020 Oct 15;156:70-81. doi: 10.1016/j.theriogenology.2020.06.014. Epub 2020 Jun 14. Theriogenology. 2020. PMID: 32679458
-
Proteomics: a subcellular look at spermatozoa.Reprod Biol Endocrinol. 2011 Mar 22;9:36. doi: 10.1186/1477-7827-9-36. Reprod Biol Endocrinol. 2011. PMID: 21426553 Free PMC article. Review.
-
Dark side of the epididymis: tails of sperm maturation.Andrology. 2019 Sep;7(5):566-580. doi: 10.1111/andr.12641. Epub 2019 May 17. Andrology. 2019. PMID: 31102346 Review.
Cited by
-
Age-Dependent Variations in Functional Quality and Proteomic Characteristics of Canine (Canis lupus familiaris) Epididymal Spermatozoa.Int J Mol Sci. 2022 Aug 15;23(16):9143. doi: 10.3390/ijms23169143. Int J Mol Sci. 2022. PMID: 36012418 Free PMC article.
-
Current Status and Advances in Semen Preservation.Animals (Basel). 2022 Dec 28;13(1):123. doi: 10.3390/ani13010123. Animals (Basel). 2022. PMID: 36611732 Free PMC article.
-
Molecular Biomarkers of Canine Reproductive Functions.Curr Issues Mol Biol. 2024 Jun 17;46(6):6139-6168. doi: 10.3390/cimb46060367. Curr Issues Mol Biol. 2024. PMID: 38921038 Free PMC article. Review.
-
Proteome Profiling of Canine Epididymal Fluid: In Search of Protein Markers of Epididymal Sperm Motility.Int J Mol Sci. 2023 Sep 30;24(19):14790. doi: 10.3390/ijms241914790. Int J Mol Sci. 2023. PMID: 37834239 Free PMC article.
-
Integrative Assessment of Seminal Plasma Biomarkers: A Narrative Review Bridging the Gap between Infertility Research and Clinical Practice.J Clin Med. 2024 May 27;13(11):3147. doi: 10.3390/jcm13113147. J Clin Med. 2024. PMID: 38892858 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

