Characterisation of Severe Traumatic Brain Injury Severity from Fresh Cerebral Biopsy of Living Patients: An Immunohistochemical Study
- PMID: 35327320
- PMCID: PMC8945429
- DOI: 10.3390/biomedicines10030518
Characterisation of Severe Traumatic Brain Injury Severity from Fresh Cerebral Biopsy of Living Patients: An Immunohistochemical Study
Abstract
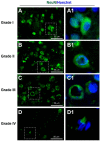
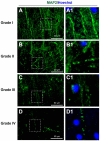
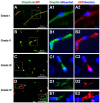
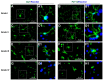
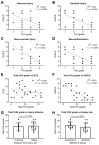
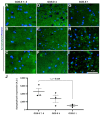
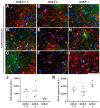
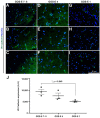
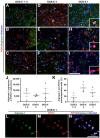
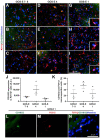
Traumatic brain injury (TBI) is an extremely complex disease and current systems classifying TBI as mild, moderate, and severe often fail to capture this complexity. Neuroimaging cannot resolve the cellular and molecular changes due to lack of resolution, and post-mortem tissue examination may not adequately represent acute disease. Therefore, we examined the cellular and molecular sequelae of TBI in fresh brain samples and related these to clinical outcomes. Brain biopsies, obtained shortly after injury from 25 living adult patients suffering severe TBI, underwent immunohistochemical analysis. There were no adverse events. Immunostaining revealed various qualitative cellular and biomolecular changes relating to neuronal injury, dendritic injury, neurovascular injury, and neuroinflammation, which we classified into 4 subgroups for each injury type using the newly devised Yip, Hasan and Uff (YHU) grading system. Based on the Glasgow Outcome Scale-Extended, a total YHU grade of ≤8 or ≥11 had a favourable and unfavourable outcome, respectively. Biomolecular changes observed in fresh brain samples enabled classification of this heterogeneous patient population into various injury severity categories based on the cellular and molecular pathophysiology according to the YHU grading system, which correlated with outcome. This is the first study investigating the acute biomolecular response to TBI.
Keywords: Glasgow Outcome Scale-Extended; brain biopsy; dendritic injury; neuroinflammation; neuronal injury; neurovasculature; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[The prognostic value of mri-classification of traumatic brain lesions level and localization depending on neuroimaging timing].Zh Vopr Neirokhir Im N N Burdenko. 2019;83(4):46-55. doi: 10.17116/neiro20198304146. Zh Vopr Neirokhir Im N N Burdenko. 2019. PMID: 31577269 Russian.
-
Impact of Glasgow Coma Scale score and pupil parameters on mortality rate and outcome in pediatric and adult severe traumatic brain injury: a retrospective, multicenter cohort study.J Neurosurg. 2017 Mar;126(3):760-767. doi: 10.3171/2016.1.JNS152385. Epub 2016 Apr 1. J Neurosurg. 2017. PMID: 27035177
-
An evaluation of outcomes in patients with traumatic brain injury at a referral hospital in Tanzania: evidence from a survival analysis.Neurosurg Focus. 2019 Nov 1;47(5):E6. doi: 10.3171/2019.7.FOCUS19316. Neurosurg Focus. 2019. PMID: 31675716 Free PMC article.
-
[Mild traumatic brain injury and postconcussive syndrome: a re-emergent questioning].Encephale. 2012 Sep;38(4):329-35. doi: 10.1016/j.encep.2011.07.003. Epub 2011 Aug 31. Encephale. 2012. PMID: 22980474 Review. French.
-
Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae.Pituitary. 2019 Jun;22(3):270-282. doi: 10.1007/s11102-019-00957-9. Pituitary. 2019. PMID: 30929221 Review.
Cited by
-
Advances in Visualizing Microglial Cells in Human Central Nervous System Tissue.Biomolecules. 2022 Apr 19;12(5):603. doi: 10.3390/biom12050603. Biomolecules. 2022. PMID: 35625531 Free PMC article. Review.
-
Antioxidant Therapeutic Strategies in Neurodegenerative Diseases.Int J Mol Sci. 2022 Aug 19;23(16):9328. doi: 10.3390/ijms23169328. Int J Mol Sci. 2022. PMID: 36012599 Free PMC article. Review.
-
Serum amyloid P component accumulates and persists in neurones following traumatic brain injury.Open Biol. 2023 Dec;13(12):230253. doi: 10.1098/rsob.230253. Epub 2023 Dec 6. Open Biol. 2023. PMID: 38052249 Free PMC article.
-
Omega-3 Fatty Acids and Traumatic Injury in the Adult and Immature Brain.Nutrients. 2024 Nov 30;16(23):4175. doi: 10.3390/nu16234175. Nutrients. 2024. PMID: 39683568 Free PMC article. Review.
-
Advances Research in Traumatic Encephalopathy.Biomedicines. 2022 Sep 15;10(9):2287. doi: 10.3390/biomedicines10092287. Biomedicines. 2022. PMID: 36140388 Free PMC article.
References
LinkOut - more resources
Full Text Sources

