Mitochondrial Determinants of Anti-Cancer Drug-Induced Cardiotoxicity
- PMID: 35327322
- PMCID: PMC8945454
- DOI: 10.3390/biomedicines10030520
Mitochondrial Determinants of Anti-Cancer Drug-Induced Cardiotoxicity
Abstract
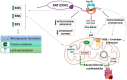
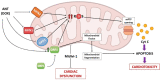
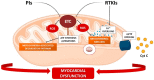
Mitochondria are key organelles for the maintenance of myocardial tissue homeostasis, playing a pivotal role in adenosine triphosphate (ATP) production, calcium signaling, redox homeostasis, and thermogenesis, as well as in the regulation of crucial pathways involved in cell survival. On this basis, it is not surprising that structural and functional impairments of mitochondria can lead to contractile dysfunction, and have been widely implicated in the onset of diverse cardiovascular diseases, including ischemic cardiomyopathy, heart failure, and stroke. Several studies support mitochondrial targets as major determinants of the cardiotoxic effects triggered by an increasing number of chemotherapeutic agents used for both solid and hematological tumors. Mitochondrial toxicity induced by such anticancer therapeutics is due to different mechanisms, generally altering the mitochondrial respiratory chain, energy production, and mitochondrial dynamics, or inducing mitochondrial oxidative/nitrative stress, eventually culminating in cell death. The present review summarizes key mitochondrial processes mediating the cardiotoxic effects of anti-neoplastic drugs, with a specific focus on anthracyclines (ANTs), receptor tyrosine kinase inhibitors (RTKIs) and proteasome inhibitors (PIs).
Keywords: anticancer therapy; cardiotoxicity; heart failure; mitochondrial function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

