Fibrin, Bone Marrow Cells and Macrophages Interactively Modulate Cardiomyoblast Fate
- PMID: 35327330
- PMCID: PMC8945703
- DOI: 10.3390/biomedicines10030527
Fibrin, Bone Marrow Cells and Macrophages Interactively Modulate Cardiomyoblast Fate
Abstract
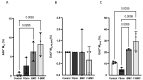
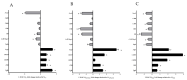
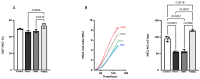
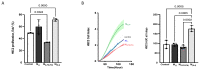
Interactions between macrophages, cardiac cells and the extracellular matrix are crucial for cardiac repair following myocardial infarction (MI). We hypothesized that cell-based treatments might modulate these interactions. After validating that bone marrow cells (BMC) associated with fibrin lowered the infarct extent and improved cardiac function, we interrogated the influence of fibrin, as a biologically active scaffold, on the secretome of BMC and the impact of their association on macrophage fate and cardiomyoblast proliferation. In vitro, BMC were primed with fibrin (F-BMC). RT-PCR and proteomic analyses showed that fibrin profoundly influenced the gene expression and the secretome of BMCs. Consequently, the secretome of F-BMC increased the spreading of cardiomyoblasts and showed an alleviated immunomodulatory capacity. Indeed, the proliferation of anti-inflammatory macrophages was augmented, and the phenotype of pro-inflammatory switched as shown by downregulated Nos2, Il6 and IL1b and upregulated Arg1, CD163, Tgfb and IL10. Interestingly, the secretome of F-BMC educated-macrophages stimulated the incorporation of EdU in cardiomyoblasts. In conclusion, our study provides evidence that BMC/fibrin-based treatment improved cardiac structure and function following MI. In vitro proofs-of-concept reveal that the F-BMC secretome increases cardiac cell size and promotes an anti-inflammatory response. Thenceforward, the F-BMC educated macrophages sequentially stimulated cardiac cell proliferation.
Keywords: cell communication; cell priming; fibrin; inflammation; macrophages; secretome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Improvement in cardiac function after bone marrow cell thearpy is associated with an increase in myocardial inflammation.Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H43-50. doi: 10.1152/ajpheart.00613.2008. Epub 2008 Nov 14. Am J Physiol Heart Circ Physiol. 2009. PMID: 19011044
-
Extracellular high mobility group box 1 plays a role in the effect of bone marrow mononuclear cell transplantation for heart failure.PLoS One. 2013 Oct 18;8(10):e76908. doi: 10.1371/journal.pone.0076908. eCollection 2013. PLoS One. 2013. PMID: 24204700 Free PMC article.
-
YAP/TAZ deficiency reprograms macrophage phenotype and improves infarct healing and cardiac function after myocardial infarction.PLoS Biol. 2020 Dec 2;18(12):e3000941. doi: 10.1371/journal.pbio.3000941. eCollection 2020 Dec. PLoS Biol. 2020. PMID: 33264286 Free PMC article.
-
Cardiac repair with adult bone marrow-derived cells: the clinical evidence.Antioxid Redox Signal. 2009 Aug;11(8):1865-82. doi: 10.1089/ars.2009.2462. Antioxid Redox Signal. 2009. PMID: 19203221 Free PMC article. Review.
-
[Autologous bone marrow cell infusion therapy for liver cirrhosis--now and future].Rinsho Byori. 2011 Dec;59(12):1092-8. Rinsho Byori. 2011. PMID: 22338911 Review. Japanese.
Cited by
-
Distinct Hemostasis and Blood Composition in Spiny Mouse Acomys cahirinus.Int J Mol Sci. 2024 Nov 29;25(23):12867. doi: 10.3390/ijms252312867. Int J Mol Sci. 2024. PMID: 39684578 Free PMC article.
-
The Tissue Engineering Revolution: From Bench Research to Clinical Reality.Biomedicines. 2024 Feb 18;12(2):453. doi: 10.3390/biomedicines12020453. Biomedicines. 2024. PMID: 38398055 Free PMC article.
-
Macrophages in the Context of Muscle Regeneration and Duchenne Muscular Dystrophy.Int J Mol Sci. 2024 Sep 27;25(19):10393. doi: 10.3390/ijms251910393. Int J Mol Sci. 2024. PMID: 39408722 Free PMC article. Review.
-
Live-imaging studies reveal how microclots and the associated inflammatory response enhance cancer cell extravasation.J Cell Sci. 2023 Sep 15;136(18):jcs261225. doi: 10.1242/jcs.261225. Epub 2023 Sep 28. J Cell Sci. 2023. PMID: 37671502 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

