The Proteome of Antibody-Mediated Rejection: From Glomerulitis to Transplant Glomerulopathy
- PMID: 35327371
- PMCID: PMC8945687
- DOI: 10.3390/biomedicines10030569
The Proteome of Antibody-Mediated Rejection: From Glomerulitis to Transplant Glomerulopathy
Abstract
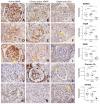
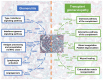
Antibody-mediated rejection (ABMR) is the leading cause of allograft failure in kidney transplantation. Its histological hallmark is represented by lesions of glomerulitis i.e., inflammatory cells within glomeruli. Current therapies for ABMR fail to prevent chronic allograft damage i.e., transplant glomerulopathy, leading to allograft loss. We used laser microdissection of glomeruli from formalin-fixed allograft biopsies combined with mass spectrometry-based proteomics to describe the proteome modification of 11 active and 10 chronic active ABMR cases compared to 8 stable graft controls. Of 1335 detected proteins, 77 were deregulated in glomerulitis compared to stable grafts, particularly involved in cellular stress mediated by interferons type I and II, leukocyte activation and microcirculation remodeling. Three proteins extracted from this protein profile, TYMP, WARS1 and GBP1, showed a consistent overexpression by immunohistochemistry in glomerular endothelial cells that may represent relevant markers of endothelial stress during active ABMR. In transplant glomerulopathy, 137 proteins were deregulated, which favor a complement-mediated mechanism, wound healing processes through coagulation activation and ultimately a remodeling of the glomerular extracellular matrix, as observed by light microscopy. This study brings novel information on glomerular proteomics of ABMR in kidney transplantation, and highlights potential targets of diagnostic and therapeutic interest.
Keywords: antibody-mediated rejection; glomerulus; kidney transplantation; proteomics; transplant glomerulopathy.
Conflict of interest statement
The authors of this manuscript have no conflict of interest to disclose.
Figures



References
-
- Sellarés J., de Freitas D.G., Mengel M., Reeve J., Einecke G., Sis B., Hidalgo L.G., Famulski K., Matas A., Halloran P.F. Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am. J. Transplant. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. - DOI - PubMed
-
- Loupy A., Haas M., Roufosse C., Naesens M., Adam B., Afrouzian M., Akalin E., Alachkar N., Bagnasco S., Becker J.U., et al. The banff 2019 kidney meeting report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 2020;20:2318–2331. doi: 10.1111/ajt.15898. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

