Placental Insulin Receptor Transiently Regulates Glucose Homeostasis in the Adult Mouse Offspring of Multiparous Dams
- PMID: 35327377
- PMCID: PMC8945682
- DOI: 10.3390/biomedicines10030575
Placental Insulin Receptor Transiently Regulates Glucose Homeostasis in the Adult Mouse Offspring of Multiparous Dams
Abstract
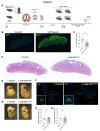
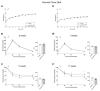
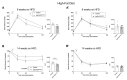
In pregnancies complicated by maternal obesity and gestational diabetes mellitus, there is strong evidence to suggest that the insulin signaling pathway in the placenta may be impaired. This may have potential effects on the programming of the metabolic health in the offspring; however, a direct link between the placental insulin signaling pathway and the offspring health remains unknown. Here, we aimed to understand whether specific placental loss of the insulin receptor (InsR) has a lasting effect on the offspring health in mice. Obesity and glucose homeostasis were assessed in the adult mouse offspring on a normal chow diet (NCD) followed by a high-fat diet (HFD) challenge. Compared to their littermate controls, InsR KOplacenta offspring were born with normal body weight and pancreatic β-cell mass. Adult InsR KOplacenta mice exhibited normal glucose homeostasis on an NCD. Interestingly, under a HFD challenge, adult male InsR KOplacenta offspring demonstrated lower body weight and a mildly improved glucose homeostasis associated with parity. Together, our data show that placenta-specific insulin receptor deletion does not adversely affect offspring glucose homeostasis during adulthood. Rather, there may potentially be a mild and transient protective effect in the mouse offspring of multiparous dams under the condition of a diet-induced obesogenic challenge.
Keywords: fetal programming; gestational diabetes; multiparity; obesity; placental insulin receptor; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Disruption of O-Linked N-Acetylglucosamine Signaling in Placenta Induces Insulin Sensitivity in Female Offspring.Int J Mol Sci. 2021 Jun 28;22(13):6918. doi: 10.3390/ijms22136918. Int J Mol Sci. 2021. PMID: 34203166 Free PMC article.
-
The loss of ERE-dependent ERα signaling potentiates the effects of maternal high-fat diet on energy homeostasis in female offspring fed an obesogenic diet.J Dev Orig Health Dis. 2020 Jun;11(3):285-296. doi: 10.1017/S2040174419000515. Epub 2019 Sep 23. J Dev Orig Health Dis. 2020. PMID: 31543088 Free PMC article.
-
Impact of placental mTOR deficiency on peripheral insulin signaling in adult mice offspring.J Mol Endocrinol. 2023 Oct 18;71(4):e230035. doi: 10.1530/JME-23-0035. Print 2023 Nov 1. J Mol Endocrinol. 2023. PMID: 37855320 Free PMC article.
-
Placental mTOR complex 1 regulates fetal programming of obesity and insulin resistance in mice.JCI Insight. 2021 Jul 8;6(13):e149271. doi: 10.1172/jci.insight.149271. JCI Insight. 2021. PMID: 34032632 Free PMC article.
-
Placental mTOR Signaling and Sexual Dimorphism in Metabolic Health across the Lifespan of Offspring.Children (Basel). 2021 Oct 26;8(11):970. doi: 10.3390/children8110970. Children (Basel). 2021. PMID: 34828683 Free PMC article. Review.
Cited by
-
Diabetes: A Multifaceted Disorder.Biomedicines. 2022 Jul 14;10(7):1698. doi: 10.3390/biomedicines10071698. Biomedicines. 2022. PMID: 35885003 Free PMC article.
-
RISING STARS: Mechanistic insights into maternal-fetal cross talk and islet beta-cell development.J Endocrinol. 2023 Nov 8;259(3):e230069. doi: 10.1530/JOE-23-0069. Print 2023 Dec 1. J Endocrinol. 2023. PMID: 37855321 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

