Influence of Fetomaternal Microchimerism on Maternal NK Cell Reactivity against the Child's Leukemic Blasts
- PMID: 35327405
- PMCID: PMC8945103
- DOI: 10.3390/biomedicines10030603
Influence of Fetomaternal Microchimerism on Maternal NK Cell Reactivity against the Child's Leukemic Blasts
Abstract
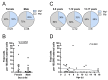
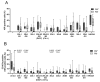
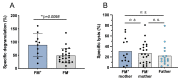
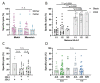
Persistence of fetal cells in the circulation of the mother (fetal microchimerism, FM) is associated with increased survival and reduced relapse of children with leukemia receiving a haploidentical hematopoietic stem cell transplantation (hHSCT). NK cells play an important role in maternal tolerance towards the unborn child. In this study, 70 mother-child pairs were prospectively analyzed for the occurrence of FM, KIR genotype and HLA-C type. We found that occurrence and level of FM were influenced by three maternal genetic factors: presence of an HLA-C1 allele, absence of KIR2DL3 and presence of a cen-B/B motif. Furthermore, an HLA-C match between mother and child favored persistence of FM. NK cells from FM+ mothers showed a 40% higher specific degranulation against their filial leukemic blasts than NK cells from FM- mothers, suggesting the presence of educated maternal NK cells. Nevertheless, cytotoxicity of parental NK cells against filial leukemic blasts was independent of KIR genetics (haplotype, B content score, centromeric and telomeric KIR gene regions) and independent of FM, indicating that additional immune effector mechanisms contribute to the beneficial effect of persisting FM in hHSCT.
Keywords: HLA; KIR; NK cell alloreactivity; fetal microchimerism; haploidentical stem cell transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Donor selection for killer immunoglobulin-like receptors B haplotype of the centromeric motifs can improve the outcome after HLA-identical sibling hematopoietic stem cell transplantation.Biol Blood Marrow Transplant. 2014 Jan;20(1):98-105. Biol Blood Marrow Transplant. 2014. PMID: 24516895
-
The beneficial role of inhibitory KIR genes of HLA class I NK epitopes in haploidentically mismatched stem cell allografts may be masked by residual donor-alloreactive T cells causing GVHD.Tissue Antigens. 2004 Mar;63(3):204-11. doi: 10.1111/j.0001-2815.2004.00182.x. Tissue Antigens. 2004. PMID: 14989709
-
Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity.Blood. 2009 Mar 26;113(13):3119-29. doi: 10.1182/blood-2008-06-164103. Epub 2008 Oct 22. Blood. 2009. PMID: 18945967
-
[KIRs on human NK cells and in relation with HLA class I antigen--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006 Jun;14(3):627-30. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006. PMID: 16800958 Review. Chinese.
-
Reduction of Relapse after Unrelated Donor Stem Cell Transplantation by KIR-Based Graft Selection.Front Immunol. 2017 Feb 8;8:41. doi: 10.3389/fimmu.2017.00041. eCollection 2017. Front Immunol. 2017. PMID: 28228753 Free PMC article. Review.
Cited by
-
Clinical relevance of feto-maternal microchimerism in (hematopoietic stem cell) transplantation.Semin Immunopathol. 2024 Dec 7;47(1):4. doi: 10.1007/s00281-024-01028-3. Semin Immunopathol. 2024. PMID: 39644358 Free PMC article. Review.
References
-
- Stern M., Ruggeri L., Mancusi A., Bernardo M.E., de Angelis C., Bucher C., Locatelli F., Aversa F., Velardi A. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990–2995. doi: 10.1182/blood-2008-01-135285. - DOI - PMC - PubMed
-
- Ruggeri L., Eikema D.J., Bondanza A., Noviello M., van Biezen A., de Wreede L.C., Crucitti L., Vago L., Ciardelli S., Bader P., et al. Mother donors improve outcomes after HLA haploidentical transplantation: A Study by the Cellular Therapy and Immunobiology Working Party of the EBMT. Transplant. Cell. Ther. 2022 doi: 10.1016/j.jtct.2022.01.001. in press. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

