Isolation of Functional SARS-CoV-2 Antigen-Specific T-Cells with Specific Viral Cytotoxic Activity for Adoptive Therapy of COVID-19
- PMID: 35327433
- PMCID: PMC8944951
- DOI: 10.3390/biomedicines10030630
Isolation of Functional SARS-CoV-2 Antigen-Specific T-Cells with Specific Viral Cytotoxic Activity for Adoptive Therapy of COVID-19
Abstract
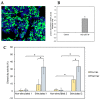
In order to demonstrate the feasibility of preparing clinical-grade SARS-CoV-2-specific T-cells from convalescent donors and the ability of these cells to neutralize the virus in vitro, we used blood collected from two COVID-19 convalescent donors (before and after vaccination) that was stimulated with specific SARS-CoV-2 peptides followed by automated T-cell isolation using the CliniMacs Prodigy medical device. To determine cytotoxic activity, HEK 293T cells were transfected to express the SARS-CoV-2 M protein, mimicking SARS-CoV-2 infection. We were able to quickly and efficiently isolate SARS-CoV-2-specific T lymphocytes from both donors before and after they received the Pfizer-BioNTech vaccine. Althoughbefore vaccination, the final product contained up to 7.42% and 30.19% of IFN-γ+ CD3+ T-cells from donor 1 and donor 2, respectively, we observed an enrichment of the IFN-γ+ CD3+ T-cells after vaccination, reaching 70.47% and 42.59%, respectively. At pre-vaccination, the isolated SARS-CoV-2-specific T-cells exhibited cytotoxic activity that was significantly higher than that of unstimulated controls (donor 2: 15.41%, p-value 3.27 × 10-3). The cytotoxic activity of the isolated SARS-CoV-2-specific T-cells also significantly increased after vaccination (donor 1: 32.71%, p-value 1.44 × 10-5; donor 2: 33.38%, p-value 3.13 × 10-6). In conclusion, we demonstrated that SARS-CoV-2-specific T-cells can quickly and efficiently be stimulated from the blood of convalescent donors using SARS-CoV-2-specific peptides followed by automated isolation. Vaccinated convalescent donors have a higher percentage of SARS-CoV-2-specific T-cells and may be more suitable as donors. Although further studies are needed to assess the clinical utility of the functional isolated SARS-CoV-2-specific T-cells in patients, previous studies using the same stimulation and isolation methods applied to other pathologies support this idea.
Keywords: M protein; SARS-CoV-2; T-lymphocytes; cytotoxicity.
Conflict of interest statement
P.P.-R. is a founder and shareholder of Vaxdyn, S.L., a biotechnology company developing vaccines. D.L. works for Miltenyi Biotech. The rest of the authors declare no conflict of interest.
Figures

References
-
- Siddell S., Ziebuhr J., Snijder E. Coronaviruses, Toroviruses, and Arteriviruses. Hodder Arnold; London, UK: 2005. pp. 823–856.
-
- Gerotziafas G.T., Catalano M., Theodorou Y., Van Dreden P., Marechal V., Spyropoulos A.C., Carter C., Jabeen N., Harenberg J., Elalamy I., et al. The COVID-19 Pandemic and the Need for an Integrated and Equitable Approach: An International Expert Consensus Paper. Thromb. Haemost. 2021;121:992–1007. doi: 10.1055/a-1535-8807. - DOI - PMC - PubMed
-
- Jagadeesh A., Prathyusha A.M.V.N., Sheela G.M., Bramhachari P.V. T-cells in Viral Infections: The Myriad Flavours of Antiviral Immunity. In: Bramhachari P.V., editor. Dynamics of Immune Activation in Viral Diseases. Springer; Singapore: 2020. pp. 139–148.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

