SARS-CoV-2 mRNA Vaccine-Induced Cellular and Humoral Immunity in Hemodialysis Patients
- PMID: 35327438
- PMCID: PMC8945424
- DOI: 10.3390/biomedicines10030636
SARS-CoV-2 mRNA Vaccine-Induced Cellular and Humoral Immunity in Hemodialysis Patients
Abstract
Background/aims: Chronic kidney disease CKD patients on intermittent hemodialysis IHD are exposed to SARS-CoV-2 infection and carry a risk of developing severe symptoms. The aim of this study was to evaluate the humoral and cellular immunity induced by two doses of mRNA vaccines, the Pfizer-BioNTech (Comirnaty) COVID-19 Vaccine and the Moderna (mRNA-1273) COVID-19 vaccine.
Patients and methods: The study included 281 patients from five dialysis centers in northern Poland. Within 2 weeks prior to the first dose of the vaccine, a blood sample was collected for an evaluation of SARS-CoV-2 antibodies. Thirty to forty-five days after the second dose of the vaccine, a blood sample was taken to evaluate humoral and cellular response.
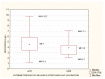
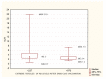
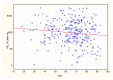
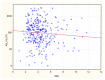
Results: Patients with stage 5 CKD on IHD were characterized by a considerable SARS-CoV-2 vaccine-induced seroconversion rate. The strongest factors influencing the antibodies AB level after vaccination were a pre-vaccination history of SARS-CoV-2 infection, age, the neutrophil-to-lymphocyte ratio NLR, neutrophil absolute count, and the hemoglobin level. Cellular immunity was higher in patients with a pre-vaccination history of SARS-CoV-2 infection. Cellular immunity depended on the albumin level. Positive cellular response to vaccination was a positive factor reducing all-cause mortality, except for COVID-19 mortality (no such deaths were reported during our follow-up). Cellular immunity and humoral immunity were positively mutually dependent. High levels of albumin and hemoglobin, low neutrophil count, and a reduced NLR, translated into better response to vaccination.
Conclusions: Patients with stage 5 CKD on IHD were characterized by a considerable SARS-CoV-2 vaccine-induced seroconversion rate and a good rate of cellular immunity. The factors that change with exacerbating inflammation and malnutrition (albumin, hemoglobin, neutrophil count, the NLR) affected the efficacy of the vaccination.
Keywords: SARS-CoV-2; cellular immunity; humoral immunity; mRNA vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



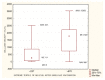
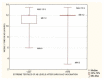

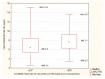
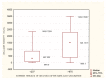







References
-
- Inchingolo A.D., Inchingolo A.M., Bordea I.R., Malcangi G., Xhajanka E., Scarano A., Lorusso F., Farronato M., Tartaglia G.M., Isacco C.G., et al. SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms. 2021;9:793. doi: 10.3390/microorganisms9040793. - DOI - PMC - PubMed
-
- Bordea I.R., Candrea S., Sălăgean T., Pop I.D., Lucaciu O., Ilea A., Manole M., Băbțan A.-M., Sirbu A., Hanna R. Impact of COVID-19 Pandemic on Healthcare Professionals and Oral Care Operational Services: A Systemic Review. Risk Manag. Heal. Policy. 2021;14:453–463. doi: 10.2147/RMHP.S284557. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

