The Antiplatelet Action of S-Nitroso Human Serum Albumin in Whole Blood
- PMID: 35327451
- PMCID: PMC8945101
- DOI: 10.3390/biomedicines10030649
The Antiplatelet Action of S-Nitroso Human Serum Albumin in Whole Blood
Abstract
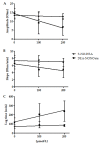
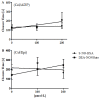
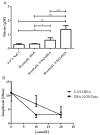
Nitric oxide donors (NO-donors) have been shown to have therapeutic potential (e.g., ischemia/reperfusion injury). However, due to their release rate/antiplatelet properties, they may cause bleeding in patients. We therefore studied the antiplatelet effects of the two different NO-donors, i.e., S-NO-Human Serum Albumin (S-NO-HSA) and Diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (DEA-NONOate) in whole blood (WB) samples. WB samples were spiked with S-NO-HSA or DEA-NONOate (100 µmol/L or 200 µmol/L), and the NO release rate (nitrite/nitrate levels via HPLC) and antiplatelet efficacy (impedance aggregometry, platelet function analyzer, Cone-and-platelet analyzer, thrombelastometry) were assessed. S-NO-HSA had a significantly lower NO release compared to equimolar concentrations of DEA-NONOate. Virtually no antiplatelet action of S-NO-HSA was observed in WB samples, whereas DEA-NONOate significantly attenuated platelet function in WB. Impedance aggregometry measurements revealed that Amplitudes (slope: -0.04022 ± 0.01045 ohm/µmol/L, p = 0.008) and Lag times (slope: 0.6389 ± 0.2075 s/µmol/L, p = 0.0051) were dose-dependently decreased and prolonged by DEA-NONOate. Closure times (Cone-and-platelet analyzer) were dose-dependently prolonged (slope: 0.3738 ± 0.1403 s/µmol/L, p = 0.0174 with collagen/ADP coating; slope: -0.5340 ± 0.1473 s/µmol/L, p = 0.0019 with collagen/epinephrine coating) by DEA-NONOate. These results in WB further support the pharmacological potential of S-NO-HSA as an NO-donor due to its ability to presumably prevent bleeding events even at high concentrations up to 200 µmol/L.
Keywords: impedance aggregometry; nitric oxide donors; platelet function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Moncada S., Palmer R.M., Higgs E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. - PubMed
LinkOut - more resources
Full Text Sources

