Inorganic Polymeric Materials for Injured Tissue Repair: Biocatalytic Formation and Exploitation
- PMID: 35327460
- PMCID: PMC8945818
- DOI: 10.3390/biomedicines10030658
Inorganic Polymeric Materials for Injured Tissue Repair: Biocatalytic Formation and Exploitation
Abstract
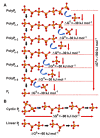
Two biocatalytically produced inorganic biomaterials show great potential for use in regenerative medicine but also other medical applications: bio-silica and bio-polyphosphate (bio-polyP or polyP). Biosilica is synthesized by a group of enzymes called silicateins, which mediate the formation of amorphous hydrated silica from monomeric precursors. The polymeric silicic acid formed by these enzymes, which have been cloned from various siliceous sponge species, then undergoes a maturation process to form a solid biosilica material. The second biomaterial, polyP, has the extraordinary property that it not only has morphogenetic activity similar to biosilica, i.e., can induce cell differentiation through specific gene expression, but also provides metabolic energy through enzymatic cleavage of its high-energy phosphoanhydride bonds. This reaction is catalyzed by alkaline phosphatase, a ubiquitous enzyme that, in combination with adenylate kinase, forms adenosine triphosphate (ATP) from polyP. This article attempts to highlight the biomedical importance of the inorganic polymeric materials biosilica and polyP as well as the enzymes silicatein and alkaline phosphatase, which are involved in their metabolism or mediate their biological activity.
Keywords: alkaline phosphatase; biomaterial; biosilica; coacervate; energy delivery; morphogenetic activity; nanoparticle; polyphosphate; silicatein; tissue regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The Physiological Inorganic Polymers Biosilica and Polyphosphate as Key Drivers for Biomedical Materials in Regenerative Nanomedicine.Int J Nanomedicine. 2024 Feb 8;19:1303-1337. doi: 10.2147/IJN.S446405. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38348175 Free PMC article. Review.
-
Hierarchical architecture of sponge spicules: biocatalytic and structure-directing activity of silicatein proteins as model for bioinspired applications.Bioinspir Biomim. 2016 Jul 25;11(4):041002. doi: 10.1088/1748-3190/11/4/041002. Bioinspir Biomim. 2016. PMID: 27452043
-
Biogenic inorganic polysilicates (biosilica): formation and biomedical applications.Prog Mol Subcell Biol. 2013;54:197-234. doi: 10.1007/978-3-642-41004-8_8. Prog Mol Subcell Biol. 2013. PMID: 24420715 Review.
-
Bioactive and biodegradable silica biomaterial for bone regeneration.Bone. 2014 Oct;67:292-304. doi: 10.1016/j.bone.2014.07.025. Epub 2014 Aug 1. Bone. 2014. PMID: 25088401
-
Biosilica: Molecular Biology, Biochemistry and Function in Demosponges as well as its Applied Aspects for Tissue Engineering.Adv Mar Biol. 2012;62:231-71. doi: 10.1016/B978-0-12-394283-8.00005-9. Adv Mar Biol. 2012. PMID: 22664124 Review.
Cited by
-
Functionalized Magnesium Phosphate Cement Induces In Situ Vascularized Bone Regeneration via Surface Lyophilization of Chondroitin Sulfate.Biomedicines. 2023 Dec 28;12(1):74. doi: 10.3390/biomedicines12010074. Biomedicines. 2023. PMID: 38255182 Free PMC article.
-
Biomechanical Behaviors and Degradation Properties of Multilayered Polymer Scaffolds: The Phase Space Method for Bile Duct Design and Bioengineering.Biomedicines. 2023 Mar 1;11(3):745. doi: 10.3390/biomedicines11030745. Biomedicines. 2023. PMID: 36979723 Free PMC article.
-
The Physiological Inorganic Polymers Biosilica and Polyphosphate as Key Drivers for Biomedical Materials in Regenerative Nanomedicine.Int J Nanomedicine. 2024 Feb 8;19:1303-1337. doi: 10.2147/IJN.S446405. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38348175 Free PMC article. Review.
References
-
- Hildebrand M., Lerch S.J.L., Shrestha R.P. Understanding diatom cell wall silicification—Moving forward. Front. Mar. Sci. 2018;5:125. doi: 10.3389/fmars.2018.00125. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

