Extracellular Vesicles Derived from Acidified Metastatic Melanoma Cells Stimulate Growth, Migration, and Stemness of Normal Keratinocytes
- PMID: 35327461
- PMCID: PMC8945455
- DOI: 10.3390/biomedicines10030660
Extracellular Vesicles Derived from Acidified Metastatic Melanoma Cells Stimulate Growth, Migration, and Stemness of Normal Keratinocytes
Abstract
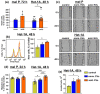
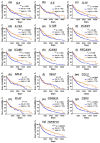
Metastatic melanoma is a highly malignant tumor. Melanoma cells release extracellular vesicles (EVs), which contribute to the growth, metastasis, and malignancy of neighboring cells by transfer of tumor-promoting miRNAs, mRNA, and proteins. Melanoma microenvironment acidification promotes tumor progression and determines EVs' properties. We studied the influence of EVs derived from metastatic melanoma cells cultivated at acidic (6.5) and normal (7.4) pH on the morphology and homeostasis of normal keratinocytes. Acidification of metastatic melanoma environment made EVs more prooncogenic with increased expression of prooncogenic mi221 RNA, stemless factor CD133, and pro-migration factor SNAI1, as well as with downregulated antitumor mir7 RNA. Incubation with EVs stimulated growth and migration both of metastatic melanoma cells and keratinocytes and changed the morphology of keratinocytes to stem-like phenotype, which was confirmed by increased expression of the stemness factors KLF and CD133. Activation of the AKT/mTOR and ERK signaling pathways and increased expression of epidermal growth factor receptor EGFR and SNAI1 were detected in keratinocytes upon incubation with EVs. Moreover, EVs reduced the production of different cytokines (IL6, IL10, and IL12) and adhesion factors (sICAM-1, sICAM-3, sPecam-1, and sCD40L) usually secreted by keratinocytes to control melanoma progression. Bioinformatic analysis revealed the correlation between decreased expression of these secreted factors and worse survival prognosis for patients with metastatic melanoma. Altogether, our data mean that metastatic melanoma EVs are important players in the transformation of normal keratinocytes.
Keywords: SNAI; adhesion factors; cancer; cytokines; extracellular vesicles; mRNA; melanoma; metastasis; miRNA; migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Extracellular Vesicles Derived from Metastatic Melanoma Cells Transfer α7-nAChR mRNA, Thus Increasing the Surface Expression of the Receptor and Stimulating the Growth of Normal Keratinocytes.Acta Naturae. 2022 Jul-Sep;14(3):95-99. doi: 10.32607/actanaturae.11734. Acta Naturae. 2022. PMID: 36348718 Free PMC article.
-
Extracellular vesicle-dependent effect of RNA-binding protein IGF2BP1 on melanoma metastasis.Oncogene. 2019 May;38(21):4182-4196. doi: 10.1038/s41388-019-0797-3. Epub 2019 Apr 1. Oncogene. 2019. PMID: 30936459 Free PMC article.
-
Melanoma-derived extracellular vesicles instigate proinflammatory signaling in the metastatic microenvironment.Int J Cancer. 2019 Nov 1;145(9):2521-2534. doi: 10.1002/ijc.32521. Epub 2019 Jul 4. Int J Cancer. 2019. PMID: 31216364
-
The Roles of Extracellular Vesicles in Malignant Melanoma.Cells. 2021 Oct 14;10(10):2740. doi: 10.3390/cells10102740. Cells. 2021. PMID: 34685720 Free PMC article. Review.
-
Extracellular Vesicles in Cancer Drug Resistance: Implications on Melanoma Therapy.Cancers (Basel). 2023 Feb 8;15(4):1074. doi: 10.3390/cancers15041074. Cancers (Basel). 2023. PMID: 36831417 Free PMC article. Review.
Cited by
-
Extracellular vesicles promote migration despite BRAF inhibitor treatment in malignant melanoma cells.Cell Commun Signal. 2024 May 22;22(1):282. doi: 10.1186/s12964-024-01660-4. Cell Commun Signal. 2024. PMID: 38778340 Free PMC article.
-
Extracellular Vesicles Derived from Metastatic Melanoma Cells Transfer α7-nAChR mRNA, Thus Increasing the Surface Expression of the Receptor and Stimulating the Growth of Normal Keratinocytes.Acta Naturae. 2022 Jul-Sep;14(3):95-99. doi: 10.32607/actanaturae.11734. Acta Naturae. 2022. PMID: 36348718 Free PMC article.
-
miR-214-Enriched Extracellular Vesicles Released by Acid-Adapted Melanoma Cells Promote Inflammatory Macrophage-Dependent Tumor Trans-Endothelial Migration.Cancers (Basel). 2022 Oct 18;14(20):5090. doi: 10.3390/cancers14205090. Cancers (Basel). 2022. PMID: 36291876 Free PMC article.
-
Selective targeting of α7 nicotinic acetylcholine receptor by synthetic peptide mimicking loop I of human SLURP-1 provides efficient and prolonged therapy of epidermoid carcinoma in vivo.Front Cell Dev Biol. 2023 Oct 3;11:1256716. doi: 10.3389/fcell.2023.1256716. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37854069 Free PMC article.
-
Contribution of Tumor-Derived Extracellular Vesicles to Malignant Transformation of Normal Cells.Bioengineering (Basel). 2022 Jun 4;9(6):245. doi: 10.3390/bioengineering9060245. Bioengineering (Basel). 2022. PMID: 35735488 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

