Role of CCR2-Positive Macrophages in Pathological Ventricular Remodelling
- PMID: 35327464
- PMCID: PMC8945438
- DOI: 10.3390/biomedicines10030661
Role of CCR2-Positive Macrophages in Pathological Ventricular Remodelling
Abstract
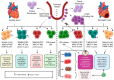
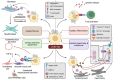
Even with recent advances in care, heart failure remains a major cause of morbidity and mortality, which urgently needs new treatments. One of the major antecedents of heart failure is pathological ventricular remodelling, the abnormal change in the size, shape, function or composition of the cardiac ventricles in response to load or injury. Accumulating immune cell subpopulations contribute to the change in cardiac cellular composition that occurs during ventricular remodelling, and these immune cells can facilitate heart failure development. Among cardiac immune cell subpopulations, macrophages that are recognized by their transcriptional or cell-surface expression of the chemokine receptor C-C chemokine receptor type 2 (CCR2), have emerged as playing an especially important role in adverse remodelling. Here, we assimilate the literature that has been generated over the past two decades describing the pathological roles that CCR2+ macrophages play in ventricular remodelling. The goal is to facilitate research and innovation efforts in heart failure therapeutics by drawing attention to the importance of studying the manner by which CCR2+ macrophages mediate their deleterious effects.
Keywords: CCR2; heart failure; inflammation; macrophage; monocyte; myocardial infarction; pressure overload; single-cell RNA sequencing; ventricular remodelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Tissue Resident CCR2- and CCR2+ Cardiac Macrophages Differentially Orchestrate Monocyte Recruitment and Fate Specification Following Myocardial Injury.Circ Res. 2019 Jan 18;124(2):263-278. doi: 10.1161/CIRCRESAHA.118.314028. Circ Res. 2019. PMID: 30582448 Free PMC article.
-
CCR2+ Monocyte-Derived Infiltrating Macrophages Are Required for Adverse Cardiac Remodeling During Pressure Overload.JACC Basic Transl Sci. 2018 Mar 14;3(2):230-244. doi: 10.1016/j.jacbts.2017.12.006. eCollection 2018 Apr. JACC Basic Transl Sci. 2018. PMID: 30062209 Free PMC article.
-
Molecular Imaging Visualizes Recruitment of Inflammatory Monocytes and Macrophages to the Injured Heart.Circ Res. 2019 Mar 15;124(6):881-890. doi: 10.1161/CIRCRESAHA.118.314030. Circ Res. 2019. PMID: 30661445 Free PMC article.
-
Recent advances targeting C-C chemokine receptor type 2 for liver diseases in monocyte/macrophage.Liver Int. 2020 Dec;40(12):2928-2936. doi: 10.1111/liv.14687. Epub 2020 Oct 21. Liver Int. 2020. PMID: 33025657 Review.
-
Different Roles of Resident and Non-resident Macrophages in Cardiac Fibrosis.Front Cardiovasc Med. 2022 Mar 7;9:818188. doi: 10.3389/fcvm.2022.818188. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35330948 Free PMC article. Review.
Cited by
-
Temporal changes in glucose metabolism reflect polarization in resident and monocyte-derived macrophages after myocardial infarction.Front Cardiovasc Med. 2023 May 5;10:1136252. doi: 10.3389/fcvm.2023.1136252. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37215542 Free PMC article.
-
Pressure overload induces ISG15 to facilitate adverse ventricular remodeling and promote heart failure.J Clin Invest. 2023 May 1;133(9):e161453. doi: 10.1172/JCI161453. J Clin Invest. 2023. PMID: 37115698 Free PMC article.
-
Concomitant Diabetes and Atrial Fibrillation: Epicardial Fat and Macrophage-Related Mechanisms.Diabetes Metab Res Rev. 2025 Jul;41(5):e70065. doi: 10.1002/dmrr.70065. Diabetes Metab Res Rev. 2025. PMID: 40587764 Free PMC article. Review.
-
Pathophysiology of Angiotensin II-Mediated Hypertension, Cardiac Hypertrophy, and Failure: A Perspective from Macrophages.Cells. 2024 Dec 4;13(23):2001. doi: 10.3390/cells13232001. Cells. 2024. PMID: 39682749 Free PMC article. Review.
-
Macrophages in the Inflammatory Phase following Myocardial Infarction: Role of Exogenous Ubiquitin.Biology (Basel). 2023 Sep 20;12(9):1258. doi: 10.3390/biology12091258. Biology (Basel). 2023. PMID: 37759657 Free PMC article. Review.
References
-
- James S.L., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., Abbastabar H., Abd-Allah F., Abdela J., Abdelalim A., et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

