Subretinal Implantation of Human Primary RPE Cells Cultured on Nanofibrous Membranes in Minipigs
- PMID: 35327471
- PMCID: PMC8945676
- DOI: 10.3390/biomedicines10030669
Subretinal Implantation of Human Primary RPE Cells Cultured on Nanofibrous Membranes in Minipigs
Abstract
Purpose: The development of primary human retinal pigmented epithelium (hRPE) for clinical transplantation purposes on biodegradable scaffolds is indispensable. We hereby report the results of the subretinal implantation of hRPE cells on nanofibrous membranes in minipigs.
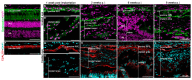
Methods: The hRPEs were collected from human cadaver donor eyes and cultivated on ultrathin nanofibrous carriers prepared via the electrospinning of poly(L-lactide-co-DL-lactide) (PDLLA). "Libechov" minipigs (12-36 months old) were used in the study, supported by preoperative tacrolimus immunosuppressive therapy. The subretinal implantation of the hRPE-nanofibrous carrier was conducted using general anesthesia via a custom-made injector during standard three-port 23-gauge vitrectomy, followed by silicone oil endotamponade. The observational period lasted 1, 2, 6 and 8 weeks, and included in vivo optical coherence tomography (OCT) of the retina, as well as post mortem immunohistochemistry using the following antibodies: HNAA and STEM121 (human cell markers); Bestrophin and CRALBP (hRPE cell markers); peanut agglutining (PNA) (cone photoreceptor marker); PKCα (rod bipolar marker); Vimentin, GFAP (macroglial markers); and Iba1 (microglial marker).
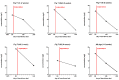
Results: The hRPEs assumed cobblestone morphology, persistent pigmentation and measurable trans-epithelial electrical resistance on the nanofibrous PDLLA carrier. The surgical delivery of the implants in the subretinal space of the immunosuppressed minipigs was successfully achieved and monitored by fundus imaging and OCT. The implanted hRPEs were positive for HNAA and STEM121 and were located between the minipig's neuroretina and RPE layers at week 2 post-implantation, which was gradually attenuated until week 8. The neuroretina over the implants showed rosette or hypertrophic reaction at week 6. The implanted cells expressed the typical RPE marker bestrophin throughout the whole observation period, and a gradual diminishing of the CRALBP expression in the area of implantation at week 8 post-implantation was observed. The transplanted hRPEs appeared not to form a confluent layer and were less capable of keeping the inner and outer retinal segments intact. The cone photoreceptors adjacent to the implant scaffold were unchanged initially, but underwent a gradual change in structure after hRPE implantation; the retina above and below the implant appeared relatively healthy. The glial reaction of the transplanted and host retina showed Vimentin and GFAP positivity from week 1 onward. Microglial activation appeared in the retinal area of the transplant early after the surgery, which seemed to move into the transplant area over time.
Conclusions: The differentiated hRPEs can serve as an alternative cell source for RPE replacement in animal studies. These cells can be cultivated on nanofibrous PDLLA and implanted subretinally into minipigs using standard 23-gauge vitrectomy and implantation injector. The hRPE-laden scaffolds demonstrated relatively good incorporation into the host retina over an eight-week observation period, with some indication of a gliotic scar formation, and a likely neuroinflammatory response in the transplanted area despite the use of immunosuppression.
Keywords: human primary RPE; minipigs; nanofibrous PDLLA membranes; subretinal implantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Subretinal Implantation of RPE on a Carrier in Minipigs: Guidelines for Preoperative Preparations, Surgical Techniques, and Postoperative Care.J Vis Exp. 2022 Nov 11;(189). doi: 10.3791/63505. J Vis Exp. 2022. PMID: 36440839
-
Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatán minipigs.Graefes Arch Clin Exp Ophthalmol. 2016 Aug;254(8):1553-1565. doi: 10.1007/s00417-016-3386-y. Epub 2016 Jun 22. Graefes Arch Clin Exp Ophthalmol. 2016. PMID: 27335025
-
Advantages of nanofibrous membranes for culturing of primary RPE cells compared to commercial scaffolds.Acta Ophthalmol. 2022 Aug;100(5):e1172-e1185. doi: 10.1111/aos.15034. Epub 2021 Oct 22. Acta Ophthalmol. 2022. PMID: 34687141
-
Rescue from light-induced retinal degeneration by human fetal retinal transplantation in minipigs.Curr Eye Res. 2009 Jul;34(7):523-35. doi: 10.1080/02713680902936148. Curr Eye Res. 2009. PMID: 19899965
-
[Retinal pigment epithelial cell transplantation: perspective].Nippon Ganka Gakkai Zasshi. 1996 Dec;100(12):982-1006. Nippon Ganka Gakkai Zasshi. 1996. PMID: 9022310 Review. Japanese.
Cited by
-
Electrospun poly(l-lactide-co-dl-lactide) nanofibrous scaffold as substrate for ex vivo limbal epithelial cell cultivation.Heliyon. 2024 May 10;10(10):e30970. doi: 10.1016/j.heliyon.2024.e30970. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803982 Free PMC article.
-
Progress in Stem Cells-Based Replacement Therapy for Retinal Pigment Epithelium: In Vitro Differentiation to In Vivo Delivery.Stem Cells Transl Med. 2023 Aug 16;12(8):536-552. doi: 10.1093/stcltm/szad039. Stem Cells Transl Med. 2023. PMID: 37459045 Free PMC article. Review.
-
Delivery of Human iPSC-Derived RPE Cells in Healthy Minipig Retina Results in Interaction Between Photoreceptors and Transplanted Cells.Adv Sci (Weinh). 2025 May;12(20):e2412301. doi: 10.1002/advs.202412301. Epub 2025 Apr 2. Adv Sci (Weinh). 2025. PMID: 40171949 Free PMC article.
-
Atrophic Macular Degeneration and Stem Cell Therapy: A Clinical Review.Adv Exp Med Biol. 2025;1474:105-118. doi: 10.1007/5584_2024_819. Adv Exp Med Biol. 2025. PMID: 39259423 Review.
-
Restored phagocytic ability of RPE patches derived from gene-corrected retinitis pigmentosa-hiPSCs on a biodegradable scaffold via clinical-grade protocol: Implications for autologous therapy.Genes Dis. 2025 Mar 22;12(6):101609. doi: 10.1016/j.gendis.2025.101609. eCollection 2025 Nov. Genes Dis. 2025. PMID: 40808876 Free PMC article. No abstract available.
References
-
- Cachafeiro M., Bemelmans A.P., Samardzija M., Afanasieva T., Pournaras J.-A., Grimm C., Kostic C., Philippe S., Wenzel A., Arsenijevic Y. Hyperactivation of retina by light in mice leads to photoreceptor cell death mediated by VEGF and retinal pigment epithelium permeability. Cell Death Dis. 2013;4:e781. doi: 10.1038/cddis.2013.303. - DOI - PMC - PubMed
-
- Szatmari-Toth M., Kristof E., Vereb Z., Akhtar S., Facsko A., Fesus L., Kauppinen A., Kaarniranta K., Petrovski G. Clearance of autophagy-associated dying retinal pigment epithelial cells—A possible source for inflammation in age-related macular degeneration. Cell Death Dis. 2016;7:e2367. doi: 10.1038/cddis.2016.133. - DOI - PMC - PubMed
-
- Nagymihaly R.N.Y., Ardan T., Motlik J., Eidet J.R., Moe M.C., Bergersen L.H., Lytvynchuk L., Petrovski G. Tissue Barriers in Disease, Injury and Regeneration. Elsevier; Amsterdam, The Netherlands: 2021. The retinal pigment epithelium: At the forefront of the blood-retinal barrier in physiology and disease; pp. 115–146.
LinkOut - more resources
Full Text Sources
Miscellaneous

