Expression Profile of CD157 Reveals Functional Heterogeneity of Capillaries in Human Dermal Skin
- PMID: 35327478
- PMCID: PMC8945771
- DOI: 10.3390/biomedicines10030676
Expression Profile of CD157 Reveals Functional Heterogeneity of Capillaries in Human Dermal Skin
Abstract
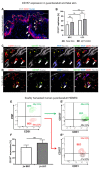
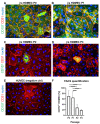
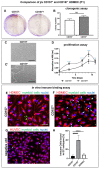
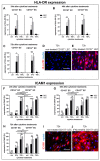
CD157 acts as a receptor, regulating leukocyte trafficking and the binding of extracellular matrix components. However, the expression pattern and the role of CD157 in human blood (BEC) and the lymphatic endothelial cells (LEC) of human dermal microvascular cells (HDMEC), remain elusive. We demonstrated constitutive expression of CD157 on BEC and LEC, in fetal and juvenile/adult skin, in situ, as well as in isolated HDMEC. Interestingly, CD157 epitopes were mostly localized on BEC, co-expressing high levels of CD31 (CD31High), as compared to CD31Low BEC, whereas the podoplanin expression level on LEC did not affect CD157. Cultured HDMEC exhibited significantly higher numbers of CD157-positive LEC, as compared to BEC. Interestingly, separated CD157- and CD157+ HDMEC demonstrated no significant differences in clonal expansion in vitro, but they showed distinct expression levels of cell adhesion molecules, before and after cytokine stimulation in vitro. In particular, we proved the enhanced and specific adherence of CD11b-expressing human blood myeloid cells to CD157+ HDMEC fraction, using an in vitro immune-binding assay. Indeed, CD157 was also involved in chemotaxis and adhesion of CD11b/c monocytes/neutrophils in prevascularized dermo-epidermal skin substitutes (vascDESS) in vivo. Thus, our data attribute specific roles to endothelial CD157, in the regulation of innate immunity during inflammation.
Keywords: CD157; CD157 receptor; angiogenesis; blood capillaries; immune cell adhesion; lymphatic capillaries; microvascular endothelial cells; myeloid cells; skin bio-engineering; vascular network formation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Role of CD200-CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation.Cells. 2022 Mar 21;11(6):1055. doi: 10.3390/cells11061055. Cells. 2022. PMID: 35326506 Free PMC article.
-
Comparison of in vivo immune responses following transplantation of vascularized and non-vascularized human dermo-epidermal skin substitutes.Pediatr Surg Int. 2017 Mar;33(3):377-382. doi: 10.1007/s00383-016-4031-x. Epub 2016 Dec 20. Pediatr Surg Int. 2017. PMID: 27999947
-
Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells.J Immunol. 1992 Jul 15;149(2):698-705. J Immunol. 1992. PMID: 1378077
-
CD157: From immunoregulatory protein to potential therapeutic target.Immunol Lett. 2019 Jan;205:59-64. doi: 10.1016/j.imlet.2018.06.007. Epub 2018 Jun 21. Immunol Lett. 2019. PMID: 29936181 Review.
-
Expression of functional melanocortin receptors and proopiomelanocortin peptides by human dermal microvascular endothelial cells.Ann N Y Acad Sci. 1999 Oct 20;885:239-53. doi: 10.1111/j.1749-6632.1999.tb08681.x. Ann N Y Acad Sci. 1999. PMID: 10816657 Review.
Cited by
-
Editorial: Revisiting immunological roles for bone marrow stromal cell antigen-1; an entero-neuro-immune regulator.Front Immunol. 2023 Jun 27;14:1239546. doi: 10.3389/fimmu.2023.1239546. eCollection 2023. Front Immunol. 2023. PMID: 37441068 Free PMC article. No abstract available.
-
Isolation and Culture of Human Dermal Fibroblasts.Methods Mol Biol. 2025;2922:75-83. doi: 10.1007/978-1-0716-4510-9_6. Methods Mol Biol. 2025. PMID: 40208528
-
CD146 expression profile in human skin and pre-vascularized dermo-epidermal skin substitutes in vivo.J Biol Eng. 2023 Jan 31;17(1):9. doi: 10.1186/s13036-023-00327-x. J Biol Eng. 2023. PMID: 36721239 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

