Human Epidural AD-MSC Exosomes Improve Function Recovery after Spinal Cord Injury in Rats
- PMID: 35327480
- PMCID: PMC8945172
- DOI: 10.3390/biomedicines10030678
Human Epidural AD-MSC Exosomes Improve Function Recovery after Spinal Cord Injury in Rats
Abstract
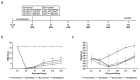
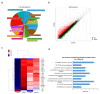
Spinal cord injury (SCI) interferes with the normal function of the autonomic nervous system by blocking circuits between the sensory and motor nerves. Although many studies focus on functional recovery after neurological injury, effective neuroregeneration is still being explored. Recently, extracellular vesicles such as exosomes have emerged as cell-free therapeutic agents owing to their ability of cell-to-cell communication. In particular, exosomes released from mesenchymal stem cells (MSCs) have the potential for tissue regeneration and exhibit therapeutic effectiveness in neurological disorders. In this study, we isolated exosomes from human epidural adipose tissue-derived MSCs (hEpi AD-MSCs) using the tangential flow filtration method. The isolated exosomes were analyzed for size, concentration, shape, and major surface markers using nanoparticle tracking analysis, transmission electron microscopy, and flow cytometry. To evaluate their effect on SCI recovery, hEpi AD-MSC exosomes were injected intravenously in SCI-induced rats. hEpi AD-MSC exosomes improved the locomotor function of SCI-induced rats. The results of histopathological and cytokine assays showed that hEpi AD-MSC exosomes regulated inflammatory response. Genetic profiling of the rat spinal cord tissues revealed changes in the expression of inflammation-related genes after exosome administration. Collectively, hEpi AD-MSC exosomes are effective in restoring spinal functions by reducing the inflammatory response.
Keywords: exosomes; extracellular vesicles; mesenchymal stem cells; spinal cord injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Mesenchymal Stem Cell-Derived Exosomes Reduce A1 Astrocytes via Downregulation of Phosphorylated NFκB P65 Subunit in Spinal Cord Injury.Cell Physiol Biochem. 2018;50(4):1535-1559. doi: 10.1159/000494652. Epub 2018 Oct 30. Cell Physiol Biochem. 2018. PMID: 30376671
-
Human Umbilical Cord Mesenchymal Stem Cells Derived Exosomes Promote Neurological Function Recovery in a Rat Spinal Cord Injury Model.Neurochem Res. 2022 Jun;47(6):1532-1540. doi: 10.1007/s11064-022-03545-9. Epub 2022 Feb 8. Neurochem Res. 2022. PMID: 35132478
-
Advances in Management of Spinal Cord Injury Using Stem Cell-derived Extracellular Vesicles: A Review Study.Basic Clin Neurosci. 2023 Jul-Aug;14(4):443-451. doi: 10.32598/bcn.2022.3430.2. Epub 2023 Jul 1. Basic Clin Neurosci. 2023. PMID: 38050575 Free PMC article. Review.
-
Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats.J Neurotrauma. 2017 Dec 15;34(24):3388-3396. doi: 10.1089/neu.2017.5063. Epub 2017 Aug 18. J Neurotrauma. 2017. PMID: 28665182
-
Therapeutic Potential of Mesenchymal Stem Cells (MSCs) and MSC-Derived Extracellular Vesicles for the Treatment of Spinal Cord Injury.Int J Mol Sci. 2021 Dec 20;22(24):13672. doi: 10.3390/ijms222413672. Int J Mol Sci. 2021. PMID: 34948463 Free PMC article. Review.
Cited by
-
Applications of Stem Cell-Derived Extracellular Vesicles in Nerve Regeneration.Int J Mol Sci. 2024 May 28;25(11):5863. doi: 10.3390/ijms25115863. Int J Mol Sci. 2024. PMID: 38892052 Free PMC article. Review.
-
Extracellular Vesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells Suppress RANKL-Induced Osteoclast Differentiation via miR122-5p.Biochem Genet. 2024 Aug;62(4):2830-2852. doi: 10.1007/s10528-023-10569-5. Epub 2023 Nov 29. Biochem Genet. 2024. PMID: 38017286
-
The involvement of epidural fat in ossification of the ligamentum flavum: From the perspective of exosomal proteome.Heliyon. 2024 Jul 22;10(15):e34755. doi: 10.1016/j.heliyon.2024.e34755. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39144971 Free PMC article.
-
Stem cell-derived exosome treatment for acute spinal cord injury: a systematic review and meta-analysis based on preclinical evidence.Front Neurol. 2025 Jan 24;16:1447414. doi: 10.3389/fneur.2025.1447414. eCollection 2025. Front Neurol. 2025. PMID: 39926016 Free PMC article.
-
Role of MS4A7 in Regulating Microglial Polarization and Neuroinflammation in Spinal Cord Injury via the cGAS-STING-NLRP3 Axis.CNS Neurosci Ther. 2025 Jun;31(6):e70390. doi: 10.1111/cns.70390. CNS Neurosci Ther. 2025. PMID: 40522023 Free PMC article.
References
-
- Saini M., Kataruka M., Gogoi B., Sharma V., Madan G.S., Sood C. Incidence of Renal Tract Abnormalities on Ultrasonography in Patients with Spinal Cord Injury: A Retrospective Pilot Study of a Military Cohort Undergoing Long-Term Institutional Rehabilitation. Asian Spine J. 2021 doi: 10.31616/asj.2020.0471. - DOI - PMC - PubMed
-
- GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:56–87. doi: 10.1016/S1474-4422(18)30415-0. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

